Content
- Characteristics of loratadine tablets 10 mg No. 30
Composition: 1 tablet contains: loratadine 10 mg.
Excipients: lactose monohydrate - 148 mg, microcrystalline cellulose - 10.
5 mg, stearic acid - 2 mg, potato starch - up to 200 mg.
Tablets are white or almost white, flat-cylindrical, scored and chamfered.
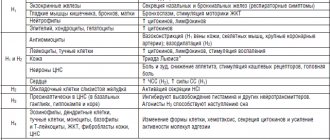
Pharmacological action: Histamine H1 receptor blocker.
Has antiallergic, antipruritic, antiexudative effects.
Reduces capillary permeability, prevents the development of tissue edema, reduces the increased contractile activity of smooth muscles caused by the action of histamine.
Indications for Use: Seasonal and year-round allergic rhinitis, conjunctivitis, acute urticaria and Quincke's edema, symptoms of histaminergy caused by the use of histaminoliberates (pseudo-allergic syndromes), allergic reactions to insect bites, complex treatment of itchy dermatoses (contact allergic dermatitis, chronic eczema).
Method of Administration: For patients over 12 years of age, the medicine is prescribed at a dose of 10 mg/day.
In patients with impaired liver function, treatment should begin with 10 mg/day.
every other day or with 5 mg/day.
daily.
In case of renal failure, as well as in elderly people, the drug is used in the standard regimen.
There is no need for dose adjustment.
The same recommendations are given in the instructions for use of Loratadine Teva, Loratadine-Stom, Loratadine-Verte and Loratadine-Stada.
The only differences are the age restrictions for drugs manufactured by Teva Pharmaceutical Industries and STADA.
They are not prescribed to children under 3 years of age.
The duration of the course is determined by the doctor depending on the characteristics of the clinical situation.
If allergies are severe, other drugs (for example, corticosteroid ointment or corticosteroid drops, immunostimulants, tear substitutes, etc.) can be used in addition to Loratadine.
facilities).
Interaction: When loratadine is used simultaneously with drugs that inhibit the isoenzymes CYP3A4 and CYP2D6 or are metabolized in the liver with their participation (including
h.
cimetidine, erythromycin, ketoconazole, quinidine, fluconazole, fluoxetine), the plasma concentrations of loratadine and/or these drugs may change.
Inducers of microsomal oxidation (phenytoin, ethanol, barbiturates, zixorin, rifampicin, phenylbutazone, tricyclic antidepressants) reduce effectiveness.
Side effects: From the digestive system: - rarely - dry mouth, nausea, vomiting, gastritis; - in some cases - liver dysfunction.
From the side of the central nervous system: rarely - increased fatigue, headache, excitability (in children).
From the cardiovascular system: rarely - tachycardia.
Allergic reactions: - rarely - skin rash; - in isolated cases - anaphylactic reactions.
Dermatological reactions: in some cases - alopecia.
Contraindications: Pregnancy, lactation, children under 2 years of age, hypersensitivity to loratadine.
Special Instructions: When using loratadine, the development of seizures cannot be completely excluded, especially in predisposed patients.
Patients with impaired renal or liver function require dosage adjustment.
Storage Conditions: Room temperature
Loratadine Farmland tablets 10 mg No. 10x1
Name
Loratadine tablet 10mg cont cell pack No. 10x1
Description
Tablets are white or white with a yellowish tint, flat-cylindrical, chamfered, with a score on one side.
Main active ingredient
Loratadine
Release form
pills
Dosage
10 mg
Indications for use
For the symptomatic treatment of allergic rhinitis and chronic idiopathic urticaria.
Directions for use and doses
Adults and children over 12 years old – 1 tablet (10 mg) 1 time per day. Children 5-12 years old weighing more than 30 kg - 1 tablet (10 mg) 1 time per day. Children 5-12 years old weighing less than 30 kg - ? tablets (5 mg) 1 time per day. For children 2-5 years old, the drug (5 mg) is prescribed in the form of syrup. The tablet dosage form is not recommended for children under six years of age. The effectiveness and safety of the drug in children under 2 years of age have not been established. In adults with impaired liver and kidney function (creatinine clearance less than 30 ml/min), the initial dose should be 1 tablet (10 mg) every other day. In children 2-5 years old with impaired liver and kidney function, the initial dose is 5 ml (in the form of syrup) every other day.
Use during pregnancy and lactation
The safety of loratadine in pregnant women has not been established, so the drug should be prescribed only if the expected benefit to the mother outweighs the potential risk to the fetus. Loratadine is excreted into breast milk, so use of the drug during breastfeeding is not recommended.
Precautionary measures
In adults with impaired liver and kidney function (creatinine clearance less than 30 ml/min), the initial dose should be 1 tablet (10 mg) every other day. In children 2-5 years old with impaired liver and kidney function, the initial dose is 5 ml (in the form of syrup) every other day. The tablet dosage form is not recommended for children under six years of age. It is recommended to discontinue treatment at least 1 week before performing a skin test for allergens. The drug contains lactose and is therefore not recommended for patients with rare hereditary cases of galactose intolerance, Lapp-lactase deficiency or glucose-galactase malabsorption.
Interaction with other drugs
With simultaneous use of the drug with ketoconazole, erythromycin, cimetidine, an increase in the concentration of loratadine in the blood plasma is observed, but without any clinical manifestations. Reduces plasma erythromycin levels by 15%. Does not potentiate the effect of alcohol on the central nervous system.
Contraindications
Hypersensitivity to the drug.
Compound
Active ingredient: loratadine – 10 mg Excipients: microcrystalline cellulose, sodium starch glycolate, magnesium stearate, sodium lauryl sulfate, lactose monohydrate.
Overdose
In case of overdose, drowsiness, tachycardia, and headache may develop. In this case, it is necessary to rinse the stomach and administer activated charcoal.
Side effect
From the nervous system: headache, drowsiness, fatigue, in 2% or less - dizziness, nervousness, insomnia, anxiety, excitability (in children), fainting, depression, pain in the ears and eyes, tinnitus. Very rarely – convulsions. From the gastrointestinal tract: dry mouth, nausea, vomiting, dyspepsia, gastritis, constipation or diarrhea, taste disturbance. From the respiratory system: nasal congestion, sneezing, sinusitis, dry nose, cough, bronchitis, upper respiratory tract infections. From the genitourinary system: change in urine color, dysmenorrhea, vaginitis, decreased libido, impotence, very rarely - edema. Allergic reactions: hyperemia, urticaria, rash, itching. From the cardiovascular system: hypertension or hypotension, palpitations, tachycardia. Other: thirst, asthenia, malaise, chills, increased sweating, weight gain.
Storage conditions
In a place protected from moisture and light at a temperature not exceeding +25°C.
Loratadine tablets 10 mg package No. 10
Loratadine Pharmland, tablets 10 mg Trade non-proprietary name: Loratadine Pharmland International non-proprietary name: Loratadine/Loratadine Release form: tablets 10 mg.
Description Tablets are white or white with a yellowish tint, flat-cylindrical, chamfered, with a score on one side.
Composition Active ingredient: loratadine - 10 mg; Excipients: microcrystalline cellulose, sodium starch glycolate (type A), magnesium stearate, sodium lauryl sulfate, lactose monohydrate.
Pharmacotherapeutic group: Antihistamines for systemic use. ATX code R06AX13.
Indications for use For the symptomatic treatment of allergic rhinitis and chronic idiopathic urticaria.
Directions for use and dosage: Adults and children over 12 years of age: 1 tablet (10 mg) 1 time per day. Children 5-12 years old weighing more than 30 kg - 1 tablet (10 mg) 1 time per day. Children 5-12 years old weighing less than 30 kg - 5 mg (in the form of syrup) 1 time per day. Tablets with a dosage of 10 mg are not recommended for this category of patients. For children 2-5 years old, the drug (5 mg) is prescribed in the form of syrup. The dosage form of the tablet is not recommended for children under six years of age. The effectiveness and safety of the drug in children under 2 years of age have not been established. In adults with impaired liver and kidney function (creatinine clearance less than 30 ml/min), the initial dose should be 1 tablet (10 mg) every other day. In children 2-5 years old with impaired liver and kidney function, the initial dose is 5 ml (in the form of syrup) every other day.
Contraindications: Known hypersensitivity to loratadine or any of the excipients. - children under 2 years of age; - lactation.
Side effects In clinical studies in a group of children 2-12 years old, the most common reactions were headache (2.7%), nervousness (2.3%), fatigue; in adolescents and adults, drowsiness was most often observed (1.2 %), headache (0.6%), increased appetite (0.5%) and insomnia (0.1%). Post-marketing studies have also identified reactions that are very rare: From the immune system: anaphylaxis. From the nervous system: dizziness. From the cardiovascular system: palpitations, tachycardia. From the gastrointestinal tract: dry mouth, nausea, gastritis. From the hepatobiliary system: liver dysfunction. From the skin and subcutaneous tissue: rash, alopecia. General disorders: fatigue, weakness.
Overdose Symptoms: drowsiness, tachycardia, headache. In case of overdose, symptomatic and supportive therapy is used throughout the required period. Treatment: it is necessary to rinse the stomach and prescribe activated charcoal. Loratadine is not eliminated by hemodialysis and it has not been established whether it can be removed by peritoneal dialysis.
Interaction with other drugs Ketoconazole, erythromycin, cimetidine, when used simultaneously with Loratadine Pharmland, increase the concentration of loratadine in the blood plasma without causing any clinical manifestations or affecting the ECG. Potential interactions may occur with all known inhibitors of CYP3A4 and CYP2D6, resulting in increased plasma levels of loratadine, which may increase the likelihood of side effects. Loratadine Farmland in therapeutic doses does not potentiate the effect of alcohol on the central nervous system.
Precautions Alcohol and tranquilizers do not potentiate the effect of Loratadine Pharmland, but it is not recommended to take it at the same time as drinking alcohol. In patients with severe liver and/or kidney disease (creatinine clearance less than 30 ml/min), the initial dose should be 5 mg per day or 10 mg every other day (1 tablet every other day). In children 2-5 years old with impaired liver and kidney function, the initial dose is 5 ml (in the form of syrup) every other day. The dosage form of the tablet is not recommended for children under six years of age. It is recommended to discontinue treatment at least 1 week before performing a skin test for allergens, because Antihistamines can prevent or reduce a reaction to an allergen. The drug contains lactose and is therefore not recommended for patients with rare hereditary cases of galactose intolerance, Lapp-lactase deficiency or glucose-galactose malabsorption.
Pregnancy and lactation The safety of loratadine in pregnant women has not been established, so the drug should be prescribed only if the expected benefit to the mother outweighs the potential risk to the fetus. No teratogenic effects were observed in animal reproductive toxicity studies. However, the duration of labor and a decrease in the viability of offspring was observed in rats at an AUC level 10 times higher than the therapeutic dose.
Effect on the ability to drive a car and work with moving mechanisms. In clinical studies, there was no effect of the drug on the patient’s reaction speed when driving a vehicle or working with moving mechanisms. However, in very rare cases, drowsiness is possible, so it is not recommended to use the drug while driving or operating other mechanisms.
Storage conditions: Protected from moisture and light at a temperature not exceeding 25C.
Shelf life: 2 years. Do not use the medicine after the date indicated on the package.
Conditions for dispensing from pharmacies Without a doctor's prescription.
Packaging 10 tablets in a blister pack made of aluminum foil and polyvinyl chloride film or 10 tablets in a polymer jar with a lid that is tensioned with first opening control, sealing agent is medical cotton wool. One can or 1 or 2 or 3 blisters along with an insert in secondary packaging.