Reception is conducted by:
Tkacheva Nina Leonidovna -
Obstetrician gynecologist of the highest category.
Make an appointment
Svitova Elena Olegovna —
Obstetrician-gynecologist. Functional diagnostics doctor. Ultrasound diagnostics doctor. Doctor of prenatal ultrasound diagnostics.
Make an appointment
Implanon is a subcutaneous contraceptive implant. Outwardly, it resembles a rod with a diameter of about 2 mm and a length of 4 cm, which releases microdoses of hormones. The package contains the product in a disposable sterile applicator for subcutaneous installation. The implant is inserted into the woman’s body for 3 years. During this period, the product reliably protects against unwanted pregnancy (effectiveness exceeds 99%).
Installation of Implanon in St. Petersburg is carried out in a medical clinic. Before the procedure, the patient undergoes a consultation with a gynecologist and the necessary examination. The implant is installed by doctors who have undergone special training. This is the key to high-quality manipulation.
What is Implanon?
Implanon is a subcutaneous hormonal releasing system. The active substance is etonogestrel. The compound is a metabolite of desogestrel, a hormone of the progestin group (often used for oral contraception). The substance is released from the implant in very low doses. However, even a small amount of the hormone is enough to suppress ovulation and change the properties of the secretion of the cervical canal. It is on these effects that the contraceptive effect of Implanon is based.
The product has a number of advantages over other types of contraception:
- does not require attention from a woman;
- can be used at any age (administration is permitted after reaching adulthood);
- provides reliable contraception for 3 years (Pearl index, that is, the number of pregnancies occurring in 100 women taking this contraceptive, is 0.005);
- installed and removed in the manipulation room;
- reversible effect (does not have a significant effect on reproductive function);
- possibility of extraction at any time by a specialist (without special training and complex manipulations).
Action of Implanon
To find out whether using Implanon for pregnancy protection is right for you, you can consult an obstetrician-gynecologist at the Zdorovye medical center network. Implanon is injected under the skin in the upper third of the shoulder exclusively by a medical specialist who knows this technique. The implant is a thin elastic silicone rod, the size of which is 4 cm in length and 2 mm in diameter, so after installation it is almost invisible. In this way, a woman can maintain the confidentiality of her contraceptive use.
Implanon's action is aimed at preventing the growth and release of eggs from the ovaries, thickening the cervical mucus and impeding the passage of sperm. Hormones constantly and continuously enter a woman’s body, so the effectiveness of the drug in preventing pregnancy is 99%.
The Implanon implant has virtually no effect on lactation, which is why many women decide to use it after childbirth. The drug can be administered 4–5 weeks after the birth of the baby.
Indications for use of Implanon
Women who are not planning a pregnancy in the next few years can have a subcutaneous implant installed. The product is ideal for preventing pregnancy in the following situations:
- young age of the patient (girls often forget to take pills and experience discomfort when using intravaginal contraceptives);
- the presence of contraindications for the use of combined contraceptives taken orally;
- diseases in which pregnancy is extremely undesirable;
- regular contraception after 40 years of age if there are contraindications to the insertion of an intrauterine device.
Preparing for a contraceptive implant
Healthy women without any complaints do not require special preparation. If the patient last visited a gynecologist more than 6 months ago, the doctor will advise you to undergo a standard examination (examination, analysis of smears for microflora and oncocytology, ultrasound of the pelvis and mammary glands). If you have health complaints or chronic diseases, it is advisable to consult with relevant specialists.
Implanon installation is planned at the beginning of the cycle (the first 5 days from the beginning of menstruation). In case of natural or artificial termination of pregnancy, the drug can be administered in the first 5 days. When switching from COCs, the implant is installed the next day after taking the last active tablet. After childbirth, Implanon is administered 1.5-4 months later (depending on the woman’s health condition).
Cost of some services in our clinic
| Medical abortion MIROPRISTONE (all medications, appointment with a gynecologist, ultrasound included) | 3300 rub. |
| Gynecologist appointment | 1200 rub. |
| Control ultrasound after medical termination of pregnancy | 1000 rub. |
| Gynecological ultrasound (pelvis) using Doppler techniques (transabdominal and transvaginal) | 1200 rub. |
VIEW ALL PRICES
UP
How is Implanon inserted and removed?
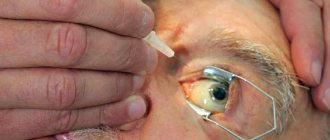
The implant is installed by a gynecologist in a medical office. At the first stage, the doctor assesses the condition of the skin in the area of injection and makes markings. Typically, the system is inserted under the skin of the inner surface of the shoulder of the left arm (for left-handers - right), 10 cm above the elbow.
The surface is treated with an antiseptic solution and an anesthetic injection is performed. At the time of the injection, the patient may experience slight discomfort, comparable to an insect bite.
Next, the doctor opens the packaging of the implant and installs it under the skin using the applicator included in the kit. The surface of the shoulder is re-disinfected, and the wound is covered with a sterile bandage. It can be removed after 6-8 hours.
After the implant expires, the woman must make an appointment with a gynecologist in the first days of the cycle. Under local anesthesia, the doctor makes a small incision above the lower pole of the rod, brings its end into the wound and removes it. If necessary, the implant can be immediately replaced with a new one.
Implanon NKST, 68 mg, 1 pc.
Before administering Implanon NKST®, pregnancy must be excluded. The gynecologist is strongly encouraged to participate in a training session to become familiar with the use of the Implanon NKST® applicator and the techniques for inserting and removing the Implanon NKST® implant. Before inserting the implant, you must carefully read the instructions for use and follow the instructions for inserting and removing the implant presented in the subsections How to insert Implanon NKST®
and
How to remove Implanon NKST®
.
How to use Implanon NKST®
The drug Implanon NKST® is a long-acting hormonal contraceptive agent. One implant is inserted subcutaneously and can remain in place for 3 years. The implant is removed no later than 3 years from the date of insertion. The woman must be informed about the possibility of removing the implant at any time, if she wishes. Your gynecologist may consider removing the implant earlier in overweight women. After removal of the implant, immediate insertion of another implant will result in continued contraceptive protection. If a woman does not want to continue using Implanon NKST®, but requires contraception, another method of contraception should be recommended.
The basis for the successful use and subsequent removal of the Implanon NKST® implant is the correct and carefully performed subcutaneous insertion of the implant in accordance with these instructions. Violation of the timing and technique of implant insertion (see subsections When to insert Implanon NKST®
,
How to administer Implanon NXT®
) can cause pregnancy.
The Implanon NKST® implant should be inserted subcutaneously, directly under the skin on the inside of the shoulder to avoid injury to large blood vessels and nerves that are located deeper in the connective tissue between the biceps and triceps muscles.
Immediately after insertion of the implant, it is necessary to palpate its presence under the skin. If the implant cannot be felt or its presence is in doubt, it is necessary to use other diagnostic methods to confirm its presence (see subsection How to administer Implanon NKST®
). Until the presence of an implant is confirmed, the woman should be advised to use a non-hormonal (barrier) method of contraception.
The packaging of Implanon NKST® contains a User Card designed to record the implant series number. The gynecologist must record the date of insertion, indicate the arm into which the implant was inserted, and the planned day of its removal in the User Card. The packaging of the drug contains stickers for the notes of the gynecologist, which indicate the series number of the implant.
When to administer Implanon NKST®
Important. Before inserting the implant, pregnancy must be ruled out.
The timing of administration depends on the woman's recent use of hormonal contraceptives as follows.
In the absence of use of contraceptive hormonal drugs in the previous month.
The implant should be inserted between day 1 (day 1 of menstrual bleeding) and day 5 of the menstrual cycle, even if menstrual bleeding is still ongoing.
If the implant is inserted correctly, an additional method of contraception is not required. If you deviate from the recommended implant insertion period, the woman should be warned about the need to use a barrier method of contraception for the next 7 days. If you had sexual intercourse during this period, pregnancy should be ruled out.
Switching from a hormonal method of contraception to Implanon NKST®
When switching from a combined method of hormonal contraception (combined oral contraceptive pill (COC), combined hormonal vaginal ring, or combined hormonal transdermal patch).
The implant should preferably be inserted on the day following the day of taking the last active tablet (the last tablet containing the active ingredients) of the COC, but no later than the day following the usual tablet-taking interval or the period during which the COC placebo tablets were taken. If a vaginal ring or transdermal patch was previously used, the implant should be inserted preferably on the day of removal, but no later than the day of the next use of the previous drug.
If the implant is inserted correctly, an additional method of contraception is not required. If you deviate from the recommended implant insertion period, the woman should be warned about the need to use a barrier method of contraception for 7 days. If you had sexual intercourse during this period, pregnancy should be ruled out.
When switching from a progestogen-only method of contraception (such as a progestogen-only pill, injection, implant, or hormonal intrauterine system (IUD)
. Since there are several types of progestogen methods, insertion of the implant should be carried out as follows:
— injectable contraceptive hormonal drugs: the implant is inserted on the day when the next injection is due;
- Progestogen-only tablets: A woman can switch from progestogen-only tablets to Implanon NKST® any day. The implant must be inserted within 24 hours after taking the last tablet;
— implant/IUD: the implant is inserted on the day of removal of the previous implant or IUD.
If the implant is inserted correctly, an additional method of contraception is not required. If you deviate from the recommended implant insertion period, the woman should be warned about the need to use a barrier method of contraception for 7 days. If you had sexual intercourse during this period, pregnancy should be ruled out.
After an abortion or miscarriage
— I trimester:
the implant must be inserted within 5 days after an abortion or miscarriage in the first trimester;
— II trimester:
the implant should be inserted between the 21st and 28th days after an abortion or miscarriage in the second trimester.
If the implant is inserted correctly, an additional method of contraception is not required. If you deviate from the recommended implant insertion period, the woman should be warned about the need to use a barrier method of contraception for 7 days. If you had sexual intercourse during this period, pregnancy should be ruled out.
After childbirth
— When breastfeeding:
the implant should be inserted at the end of the 4th week after birth (see “Use during pregnancy and lactation”). A woman should use a barrier method of contraception for 7 days after insertion of the implant. If you had sexual intercourse during this period, pregnancy should be ruled out.
— In the absence of breastfeeding:
the implant should be inserted between 21 and 28 days after birth. If the implant is inserted correctly, an additional method of contraception is not required. If you deviate from the recommended implant insertion period, the woman should be warned about the need to use a barrier method of contraception for 7 days. If you had sexual intercourse during this period, pregnancy should be ruled out.
How to administer Implanon NKST®
The basis for the successful use and subsequent removal of Implanon NKST® is the correct and carefully performed subcutaneous insertion of the implant into the non-dominant arm, in accordance with the instructions. The gynecologist and the woman must palpate to determine the presence of an implant after its insertion. The implant should be inserted directly under the skin. Too deep or incorrect insertion of the implant can be complicated by paresthesia (due to nerve damage), migration of the implant (due to IM or fascial insertion) and, in rare cases, intravascular insertion. Additionally, when the implant is inserted too deeply, it may not be palpable and localization and/or removal may be difficult.
The administration of Implanon NKST® should be performed under aseptic conditions and only by a qualified gynecologist who is familiar with the administration technique. The implant should only be inserted using a special applicator.
It is recommended that the gynecologist remain seated during the entire insertion procedure so that he can clearly see the insertion site and the movement of the needle under the skin.
The woman should lie on the examination table on her back with her non-dominant arm bent at the elbow and externally rotated so that her wrist is parallel to her ear or her arm is positioned next to her head (Figure 1).
The insertion site is located on the inside of the shoulder of the non-dominant arm, approximately 8–10 cm above the medial epicondyle of the humerus. The implant should be inserted directly under the skin to avoid damage to large vessels and nerves that are located deeper in the subcutaneous tissue in the intermuscular groove between the biceps and triceps muscles.
Make 2 marks with a sterile marker: firstly, mark the point at which the implant will be inserted, secondly, mark a point located a few centimeters proximal to the first mark (Figure 2). The second mark will subsequently serve as a guide during insertion.
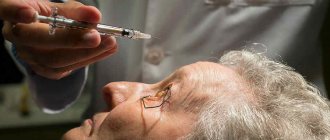
Treat the injection site with an antiseptic solution. Anesthetize the injection site (for example, using an anesthetic aerosol or injection of 2 ml of 1% lidocaine directly under the skin along the planned injection channel).
Remove the sterile disposable Implanon NKST® applicator from the blister, which contains the implant. The applicator should not be used if there is any doubt about sterility.
The applicator is held directly above the needle in the area of the textured surface and the clear protective cap is removed from the needle containing the implant (Figure 3). If the cap does not come off easily, this applicator should not be used. You can see the white-colored implant by looking at the tip of the needle. Do not touch the purple slider until the needle is fully inserted SC, as this will retract the needle and cause premature release of the implant from the applicator.
Using your free hand, use your thumb and index finger to stretch the skin around the insertion site (Figure 4).
The tip of the needle, located at approximately 30°, pierces the skin. (Figure 5).
The applicator is lowered to a horizontal position. Lifting the skin with the tip of the needle, smoothly insert the needle over its entire length (Figure 6). A slight resistance may be felt, but do not apply too much pressure. If the needle is not inserted to its full length, the implant will not be inserted properly. It is more convenient to observe the movement of the needle when the doctor sits and observes from the side, rather than looking from above. In this position, the insertion site and the movement of the needle are clearly visible.
The applicator is held in the same position after the needle has been inserted to its full length. If necessary, you can hold the applicator in the same position with your free hand during subsequent steps. Unlock the purple slider by lightly pressing it down. Move the slider all the way back until it stops (Figure 7). The implant is now under the skin and the needle is locked in the applicator. The applicator can then be removed. If the applicator is not held steady during the procedure or the purple slider is not moved all the way back, the implant will not be inserted.
After insertion, it is necessary to check the presence of the implant under the skin of the shoulder by palpation. When palpating both ends of the implant, ensure the presence of a 4 cm long rod (Figure 8).
If you cannot feel the implant or there is doubt about its presence:
— check the applicator. The needle should be fully retracted and only the purple tip of the obturator should be visible;
- to confirm the presence of an implant, you can use: two-dimensional x-ray, x-ray computed tomography (CT scan), ultrasound scan (USS) with a high-frequency ultrasound probe for linear scanning (10 MHz or more) or MRI. If the presence of an implant cannot be confirmed by these imaging methods, it is recommended to determine the concentration of etonogestrel in the woman's blood plasma. Until the presence of an implant is confirmed, a non-hormonal (barrier) contraceptive method should be used;
- apply a small adhesive tape sticker to the injection site. The woman is asked to palpate the implant;
- Apply a sterile gauze pressure bandage to reduce bruising. A woman can remove the pressure bandage after 24 hours and the small sticker from the insertion site after 3-5 days;
— fill out the User Card and give it to the woman for safekeeping. In addition, stickers are filled out and attached to the woman’s medical record;
- The applicator is intended for one-time use only and must be properly disposed of in accordance with applicable biohazard waste disposal requirements.
How to remove Implanon NKST®
Before starting the removal procedure, the gynecologist must determine the location of the implant indicated in the User Card and check it by palpation. If the implant is not palpable, then additional examination methods should be used to confirm its presence (see subsection If it is impossible to feel the implant or there are doubts about its presence
).
After localization of a non-palpable implant, the possibility of surgical removal of the implant under ultrasound control is considered.
There are rare reports of implant migration; this usually concerns a slight movement relative to the original position, with the exception of too deep insertion (see also “Special instructions”). This may make localization of the implant difficult by palpation, ultrasound, and/or MRI, and removal may require a larger incision and more time.
Removal of the implant should only be carried out under aseptic conditions by a gynecologist who is well acquainted with the removal technique.
Surgery to locate an implant without knowing its exact location is not recommended.
Removal of deep-seated implants should be done with care to avoid damage to the deep neural or vascular structures of the shoulder, and should be performed by a specialist knowledgeable about the anatomy of the shoulder.
The site of the future incision is treated with an antiseptic. Locate the implant by palpation and mark its distal end (the end closest to the elbow), such as with a sterile marker (Figure 9).
Anesthetize the area where the incision will be made, for example, 0.5–1 ml of 1% lidocaine solution (Figure 10). You should ensure that local anesthetic is injected under the implant to ensure that it remains close to the surface of the skin.
Apply pressure to the proximal end of the implant (Figure 11) to secure it; a bump may appear on the skin to mark the distal end of the implant. Starting from the distal end of the implant, a 2 mm longitudinal incision is made towards the elbow.
Gently push the implant towards the incision until the tip appears. Grasp the implant with a surgical clamp (preferably a mosquito clamp) and remove the implant (Figure 12).
If the implant is encased in connective tissue, an incision is made into the tissue envelope and then the implant is removed with a surgical forceps (Figures 13 and 14).
If the tip of the implant is not visible after the incision, a surgical clamp is carefully inserted into the incision (Figure 15).
The implant is grasped. The clamp is turned over and taken in the other hand (Figure 16).
Using the second clamp, carefully separate the tissue around the implant and grasp the implant (Figure 17). After this, the implant can be removed.
If a woman wants to continue using Implanon NKST®, a new implant can be inserted immediately, immediately after removing the old implant, into the same incision (see subsection How to replace Implanon NKST®
).
After removing the implant, close the incision with sterile strips of tissue (steri-strip) and apply an adhesive plaster sticker.
To reduce bruising, apply a sterile pressure bandage. A woman can remove the pressure bandage after 24 hours and the sticker after 3-5 days.
How to replace Implanon NKST®
Immediate replacement can be done after removal of the previous implant and is similar to the insertion procedure described in the subsection How to insert Implanon NKST®
. The new implant can be inserted into the same location and through the same incision from which the previous implant was removed. If the same incision is used to insert a new implant, anesthetize the insertion site (eg 2 ml of 1% lidocaine) by injecting directly under the skin, starting at the removal incision, along the insertion channel, and following subsequent steps in the insertion instructions. Additional information and more detailed instructions regarding insertion and removal of the implant can be obtained from the manufacturer.
Who should not use Implanon?
The following conditions are contraindications for the use of the hormonal system:
- pregnancy;
- tendency to thrombosis;
- heart attack;
- cerebrovascular accidents;
- migraine;
- oncopathology of the breast;
- severe liver disease;
- arterial hypertension;
- uterine bleeding of unknown origin;
- individual intolerance to the components of the drug.
You can install the Implanon contraceptive implant in a medical office. At the clinic, you can consult with qualified gynecologists and undergo diagnostics to exclude contraindications. The price for introducing the contraceptive Implanon does not include the cost of the drug. The product can be purchased independently at a pharmacy or ordered at a clinic. The cost of installing Implanon can be found in the corresponding section on the website or found out by phone. To make an appointment, leave your contact information in electronic form.
Prices:
Gynecology services