Indications
Ministry of Health of Russia
F32 Depressive episode
F33 Recurrent depressive disorder
F40.0 Agoraphobia
F41.0 Panic disorder [episodic paroxysmal anxiety]
F42 Obsessive-compulsive disorder
FDA recommendations
Depression
UK Medicines and Healthcare Products Regulatory Agency guidelines
Depression
Panic disorder with/without agoraphobia
Treatment regimen
Dosage and dose selection
- 20-40 mg/day
- Start with 20 mg/day, increase to 40 mg after a few weeks
- Take the daily dose at one time: morning or evening
- Treatment with a dose higher than 40 mg increases the risk of cardiac complications [1].
- If anxiety, insomnia, agitation, or akathisia occur at the beginning of treatment or after interruption of treatment, the possibility of bipolar disorder should be considered and switched to a mood stabilizer or an atypical antipsychotic
How quickly does it work?
Begins to act after 2-4 weeks
If there is no effect after 6-8 weeks, you need to increase the dose or switch to another drug
To prevent relapse, it can be taken for many years.
Expected Result
Complete remission.
After the symptoms of depression disappear, you should continue taking it for one year if this was the treatment of the first episode. If this is to treat a recurrent episode, treatment can be extended indefinitely.
Use in the treatment of anxiety is indefinite [1].
If it doesn't work
Change the dose, switch to another medicine or add an auxiliary drug;
Connect psychotherapy;
Review the diagnosis by identifying comorbid conditions;
In patients with undiagnosed bipolar affective disorder, the effectiveness of treatment may be low, in which case it is necessary to switch to a mood stabilizer [1].
How to stop taking it
There is no need to reduce it gradually, but to be sure to avoid withdrawal symptoms, you can reduce it gradually. Gradual reduction scheme: dose reduced by 50% - 3 days, again reduced by 50% - 3 days, complete cessation. If withdrawal symptoms appear, increase the dose, wait for withdrawal symptoms to subside, and continue decreasing [1].
Treatment combinations
- For insomnia: trazadone
- For fatigue, drowsiness, loss of concentration: modafinil [3].
- Combinations with other antidepressants may activate bipolar disorder and suicidal ideation
- For bipolar depression, psychotic depression, treatment-resistant depression, treatment-resistant anxiety disorder: mood stabilizers, atypical antipsychotics
- For anxiety disorder: gabapentin, tiagabine
Citalopram
Use in children and adolescents under 18 years of age
Antidepressants should not be prescribed to children and adolescents under 18 years of age. In clinical studies, children and adolescents taking antidepressants were more likely to experience suicidal behavior (suicide attempts and suicidal thoughts) and hostility (with a predominance of aggressive behavior, confrontational behavior, and irritation) than those in the placebo group.
When using drugs belonging to the SSRI therapeutic group, including citalopram, the following should be considered:
Paradoxical anxiety
Some patients with panic disorder may experience increased anxiety when starting antidepressant therapy. This paradoxical reaction usually resolves within the first two weeks after starting treatment. To reduce the likelihood of anxiogenic effects, low initial doses are recommended.
Hyponatremia
Rare cases of hyponatremia, apparently due to inadequate secretion of antidiuretic hormone (ADH), have been reported with the use of SSRIs. This reaction was generally reversible if treatment with the drug was discontinued. The risk was higher in older women.
Suicide/suicidal ideation or clinical worsening
Depression is associated with an increased risk of suicidal ideation, self-harm and suicide (suicidal events). This risk persists until stable remission develops.
Since improvement may not be observed during the first few weeks of treatment or even a longer period of time, patients should be closely monitored to ensure that such improvement is detected in a timely manner. Clinical experience shows that the risk of suicide increases in the early stages of recovery.
Other psychiatric disorders for which citalopram is prescribed may also be associated with an increased risk of suicidal events. In addition, these conditions may be a comorbidity in relation to a depressive episode. When treating patients with other mental disorders, the same precautions should be taken as when treating patients with a depressive episode.
Patients with a history of suicidal tendencies or patients with a significant level of suicidal thoughts before treatment are at greater risk for suicidal ideation or suicide attempts and should be closely monitored during treatment.
A meta-analysis of placebo-controlled clinical trials of antidepressants in adult patients with mental disorders showed that there is an increased risk of suicidal behavior in patients under 25 years of age when taking antidepressants compared with placebo.
Drug treatment of these patients, and in particular those at high risk for suicide, should be accompanied by careful monitoring, especially early in treatment and during dose changes. Patients (and caregivers) should be warned to monitor for any signs of clinical worsening, suicidal behavior or ideation, or unusual changes in behavior, and to seek immediate medical advice if these symptoms occur.
Akathisia/psychomotor restlessness
The use of drugs from the SSRI/SNRI group is associated with the development of akathisia, characterized by a feeling of subjectively unpleasant or unbearable motor restlessness, restlessness and the need to move. Often patients in this condition cannot sit or stand quietly. Most often this condition occurs during the first weeks of treatment. In patients with such symptoms, increasing the dose may cause a sharp deterioration of the condition.
Mania
Patients with bipolar affective disorder may develop a manic phase. If a manic state develops, citalopram should be discontinued.
Seizures
There is a risk of seizures when taking antidepressants. In any patient who experiences a seizure, citalopram should be discontinued. Citalopram should not be used in patients with unstable epilepsy; Controlled seizures require careful monitoring. If the frequency of seizures increases, citalopram should be discontinued.
Diabetes
In patients with diabetes mellitus, the use of SSRIs may change blood glucose concentrations. In this case, dose adjustment of insulin and/or oral hypoglycemic drugs may be required.
Serotonin syndrome
In rare cases, the development of serotonin syndrome has been reported when taking SSRIs. The development of this condition may be indicated by a combination of symptoms such as agitation, myoclonus and hyperthermia. If such phenomena occur, citalopram should be immediately discontinued and symptomatic treatment should be started.
Serotonergic drugs
Citalopram should not be used in combination with drugs that have serotonergic effects, such as sumatriptan or other triptans, tramadol, oxytriptan and tryptophan.
Bleeding
There are reports of the development of skin hemorrhages, such as ecchymosis, gynecological, gastrointestinal bleeding and other hemorrhagic complications of the skin or mucous membranes while taking SSRIs. Caution should be exercised when using SSRIs concomitantly with drugs that affect platelet function or drugs that may increase the risk of bleeding, as well as when treating patients with a history of bleeding disorders.
Electroconvulsive therapy (ECT)
Because clinical experience with the concomitant use of SSRIs and electroconvulsive therapy (ECT) is limited, caution should be used when citalopram and ECT are used concomitantly.
Reversible selective MAO A inhibitors
Concomitant use of citalopram and MAO A inhibitors is not recommended due to the risk of developing serotonin syndrome.
St. John's wort
Citalopram and preparations containing St. John's wort (Hypericum perforatum) should not be used simultaneously, because this may increase the risk of adverse reactions.
Psychosis
Treatment of psychotic patients with a depressive episode may increase the manifestation of psychotic symptoms.
Withdrawal symptoms when stopping SSRI therapy
Withdrawal symptoms occur quite often, especially when therapy is abruptly stopped.
The likelihood of withdrawal symptoms may depend on a number of factors, including the duration of treatment, the dose of the drug, and the rate at which it is tapered.
The most commonly reported symptoms were: dizziness, sensory disturbances (including paresthesia), sleep disturbances (including insomnia and vivid dreams), agitation or anxiety, nausea and/or vomiting, tremor, confusion, sweating, headache, diarrhea, rapidity palpitations, emotional lability, irritability and visual disturbances. These symptoms are usually mild or moderate in severity, but in some patients they can be severe. Typically, such manifestations develop during the first days after discontinuation of the drug, however, there are isolated reports of the development of such conditions in patients who accidentally missed taking the next dose.
In most cases, these complications resolve within 2 weeks, although in some patients symptoms may persist for 2-3 months or longer. Therefore, before ending the course of taking citalopram, it is recommended to gradually reduce the dose of the drug over a period of several weeks to several months, depending on the patient’s condition.
QT prolongation
Citalopram has been found to cause dose-dependent prolongation of the QT interval. Cases of QT prolongation and ventricular arrhythmias, including torsade de pointes
, predominantly in female patients, with hypokalemia or pre-existing QT prolongation or other cardiac disease.
The drug is recommended to be used with caution in patients with significant bradycardia, in patients who have recently suffered a myocardial infarction, or with decompensated heart failure. Electrolyte disturbances, such as hypokalemia and hypomagnesemia, increase the risk of malignant arrhythmias and should therefore be corrected before initiating citalopram therapy.
In patients with compensated heart disease, an ECG study should be performed before starting treatment.
If any signs of cardiac arrhythmias occur during treatment with citalopram, the latter should be discontinued and an ECG study performed.
Special patient groups
Patients with kidney problems
Use caution if the patient has severe kidney disease [1].
Patients with liver disease
Do not increase the dose above 20 mg [1].
Patients with heart disease
Exceeding the dose of 40 mg is dangerous [1].
Elderly patients
In patients over 60, the dose should not be raised above 20 mg;
Citalopram is one of the best-tolerated antidepressants in elderly patients [1].
Children and teenagers
- It is necessary to regularly and personally check the patient's condition, especially in the first weeks of treatment.
- Use with caution due to the risk of undiagnosed bipolar disorder and suicidality.
- Inform adults about the risks.
Pregnant
- There have been no adequate studies in pregnant women [1].
- Not recommended for pregnant women, especially in the first trimester
- All risks should be weighed and compared
- Bleeding can be expected during childbirth
Breast-feeding
- The medicine passes into breast milk.
- If the infant shows signs of irritation or sedation, discontinue feeding or citalopram
- However, treatment after childbirth may be necessary, so the risks should be weighed.
Today, antidepressants are widely used not only in psychiatric but also in neurological practice, which is primarily due to the multimodality of their action: in addition to the antidepressant effect, they have anti-anxiety, anti-panic, analgesic, sedative, stimulating and hypnotic effects. These properties are presented differently for each antidepressant. Due to the selectivity of their action, antidepressants from the group of selective serotonin reuptake inhibitors (SSRIs) have a significantly lower number of side effects and their severity compared to drugs of the previous generation (tricyclic antidepressants) and are not inferior to them in effectiveness. SSRIs are characterized by a wide spectrum of clinical action with pronounced anti-panic, anti-anxiety, and analgesic effects. One of the representatives of the SSRI group is Pram (citalopram) - a drug with proven effectiveness against panic, anxiety-depressive, and affective disorders.
Pharmacological properties
Citalopram is a racemic compound consisting of two isomers (S- and R-), which, having a pronounced ability to inhibit the reuptake of serotonin, does not bind or interacts very weakly with histamine, muscarinic and adrenergic receptors. Citalopram inhibits cytochrome P450 isoenzyme IID6 only to a very small extent and, therefore, does not interact with drugs metabolized by this enzyme. Thus, the side effects and toxic effects of this drug are very minor. Being a fat-soluble compound, citalopram easily (without connection with food intake) and quickly (after 2–4 hours) reaches its maximum concentration in biological fluids, and its lipophilicity ensures penetration through the blood-brain barrier. The half-life (T.) of the drug is about 1.5 days. Patients over the age of 65 years have a longer biological half-life (up to 3.75 days) and lower clearance values (0.08–0.3 l/min). Citalopram concentrations observed at steady state in elderly patients were almost twice those observed in younger patients receiving the same dose.
The pharmacokinetic characteristics of Prama reflect the positive properties of all SSRIs. The systemic bioavailability of the drug is 80%. Pram has linear pharmacokinetics, which provides a direct relationship between the administered dose and plasma concentration. Moreover, unlike SSRIs with nonlinear pharmacokinetics, the effect of drug dose titration can be predicted with a high degree of reliability. The equilibrium concentration of citalopram in the blood plasma is achieved regardless of the patient’s age by the end of the first week of therapy, which probably causes the development of the therapeutic effect earlier than with a number of other SSRIs. The average half-life of the active substance compared to other SSRIs (30–33 hours versus 15 hours for fluvoxamine and 4–6 days for fluoxetine) guarantees that citalopram can be taken once a day (the therapeutic dose is usually from 20 to 60 mg). A number of domestic and international studies have demonstrated the balanced action of citalopram: in addition to thymoanaleptic (i.e., antidepressant itself), the drug had anxiolytic (anti-anxiety) and activating effects. Citalopram is as effective as tricyclic and tetracyclic antidepressants against depression, but does not exhibit their inherent side effects. At the same time, Citalopram does not cause a subjective feeling of drowsiness, and also does not affect the performance of tasks associated with psychomotor functioning [7].
Indications for the use of Prama (citalopram) are depressive diseases and relapse prevention, panic disorder with and without agoraphobia, obsessive-compulsive disorder (obsessive-compulsive disorder).
Application in psychiatric practice
Since the main indications for the use of Pram (citalopram) are disorders classified according to ICD-10 in heading F “Mental disorders and behavioral disorders,” priority in research is given to psychiatrists.
A number of foreign studies (SA Montgomery et al., 1994) [22] have shown that the optimal effect when prescribing citalopram as a short-term therapy (4–6 weeks) for affective and anxiety disorders is achieved when administered at a dose of 20 mg/day, and further increase in dose to 40–80 mg/day usually does not have a significant effect on clinical improvement.
Currently, the number of studies being conducted on the basis of domestic hospitals and psychiatric hospitals is growing.
On the basis of PB No. 12 of Moscow State Scientific Center SSP named after. V.P. Serbsky studied the effectiveness of the drug Pram in patients with borderline conditions (affective and anxiety disorders) [17]. The study involved 30 outpatients over the age of 18 years who exhibited symptoms of generalized anxiety disorder or social anxiety disorder in accordance with ICD-10 and DSM-IV criteria [11, 18]. The average age of patients in the study was 29.5 years; the average duration of the disease is 4.1 years. Before starting Pram therapy, all medications were discontinued. The dynamics of the condition were assessed using the Clinical Global Impression (CGI) scale and registration of side effects. The duration of the course of therapy was 12 weeks. The examination was carried out before the start of taking Pram, on the 7th, 14, 28, 42, 56 and 84th days. All patients received the drug at an initial dose of at least 20 mg/day. Due to the insufficient effectiveness of the initial dose, in half of the cases the dosage was increased to 40 mg/day, in a small part - to 60 mg/day - subsequently. The average dosage of the drug in the study was 30 mg/day. It was revealed, according to the CGI scale used, that in 63% of cases there was a “marked improvement”, in 17% – a “minor improvement”, in 20% – “no improvement”; There were no patients with negative dynamics; there were no early dropouts due to ineffective therapy or due to adverse events in the study. Among the side effects, in a small percentage of cases, a feeling of nausea on the 3rd–6th day of therapy, insomnia disorders, and dry mouth were noted. By the 14th–28th day of treatment, all adverse events regressed. The effect of the therapy in patients with social anxiety disorders was observed at 6–7 weeks. Thus, high efficiency and good tolerability of citalopram were noted with a long course of treatment at a dosage of 20–40 mg per day, and it was stated that the drug can be used as motor therapy for patients with manifestations of generalized and social anxiety.
At the Department of Psychiatry and Narcology of Kazan State Medical University, the effectiveness of the drug Pram (citalopram) was studied in patients with non-psychotic depressive syndrome [16]. The study included 22 patients aged 30 to 55 years with this syndrome, meeting the criteria for depressive neurosis, neurotic depression or a diagnosis of dysthymia, according to ICD-10 and DSM-IV [11,18]. The criteria for excluding patients from the study were depressive syndrome of endogenous origin (schizophrenia), persistent major depression (recurrent depressive episodes), psychotic depression of other origins (organic, senile), and persons at risk of suicide. Pram was prescribed at a dose of 20 mg/day in the morning, the duration of treatment was 3 months. The patients' condition was assessed before and after the course of treatment with Pram, using the following methods: clinical-psychopathological, clinical-phenomenological with obligate questionnaires, autobiographical inventory, experimental-psychological using the full version of the MMPI, the Luscher test. The results of the study showed that Pram has a predominant effect on the anxiety component of depression, reducing affective and motor radicals, and anxious hyperphagia. After a course of therapy with Pram, positive dynamics were noted in the sleep-wake cycle: the symptom of “sensitive superficial sleep” due to anxious hyperesthesia and the symptom of “mental chewing gum” (experiencing obsessive negative events that passed during the day) when falling asleep were leveled out. Somatic manifestations associated with depressive fixation and, to a lesser extent, with sexual dysfunction regressed. To some extent, such indicators as a sense of self-worth, ideas of humiliation and the meaninglessness of existence changed. Thus, the effectiveness of citalopram is associated with a predominant effect on anxiety-depressive and other non-psychotic syndromes that have a depressive radical (obsessive-depressive, depressive-hypochondriacal disorders).
Epilepsy and depression
The prevalence of depression in different groups of patients with epilepsy ranges from 19 to 65% [10]. Symptoms that occur in a patient during an attack, persist for a long time and meet the criteria for mild to moderate depression require the prescription of antidepressants. In some cases, antiepileptic drugs themselves have side effects that include anxiety and depression, but in patients with epilepsy, depression can also be associated with seizures originating in the temporal or frontal lobes of the brain. Currently, drugs from the SSRI group are widely used in the complex treatment of depressive disorders in epilepsy. Discusses whether the patient with epilepsy has other psychiatric symptoms (eg, anxiety) that require treatment. Due to the fact that individual symptoms of depression may be part of the epileptic attack itself, this type of mental manifestations does not require treatment with antidepressants. When prescribing antidepressants to patients with epilepsy taking a basic antiepileptic drug, the following circumstances should be taken into account: simultaneous use of tricyclic antidepressants (imipramine) and valproic acid drugs can cause generalized epileptic seizures, diazepam enhances the inhibitory effect of antidepressants on the central nervous system, carbamazepine can reduce the concentration of tricyclic antidepressants (imipramine, amitriptyline, clomipramine, nortriptyline) in plasma [15]. From this point of view, citalopram has a significant advantage, since it has a slight potential for drug interaction, associated with a lower intensity of binding to plasma proteins and inhibition of isoenzymes of the cytochrome P450 system in the liver than other SSRIs. Thus, citalopram (Pram) is the drug of choice for the treatment of mild to moderate depression when combined with anticonvulsants in patients with epilepsy.
Application in neurological practice
Antidepressants are widely used in neurological practice. They have long been successfully used in the treatment of various types of depression, chronic pain syndromes, and panic attacks. In particular, drugs from the SSRI group are very popular among neurologists [7].
One of the indications for the use of citalopram is the treatment of panic disorder with and without agoraphobia. The concepts of “vegetative crisis”, “diencephalic paroxysm”, “hypothalamic crisis”, traditional for domestic neurology, have now been replaced by the term “panic attack” (PA), which is also used in cases where the attack occurs without fear and panic [6, 9 ]. According to ICD-10 [11], PA is the main manifestation of “panic disorders”, which are included in the categories of anxiety disorders (F40) and (F41). Anxiety disorders are in turn included in the class of “neurotic, stress-related and somatoform disorders” (F4).
PA is the most severe form of anxiety disorders, leading to severe maladjustment [7], and has a chronic course with exacerbations and remissions [1, 5, 14]. However, patients who come to see a neurologist with symptoms of PA can also be coded, according to ICD-10 [11], and in the heading G.90 “Disorders of the autonomic (autonomic) nervous system,” namely G.90.9 “Disorder of the autonomic ) nervous system, unspecified.” Autonomic crisis is the most striking and dramatic manifestation of vegetative dystonia syndrome.
PAs are discrete periods in which there is a sudden onset of intense anxiety, fear, or terror, often associated with a feeling of impending doom. During these attacks, symptoms such as shortness of breath, throbbing, chest pain or discomfort, a feeling of suffocation, and fear of “going crazy” or “losing control” are present [7]. Diagnostic criteria for PA are paroxysmalness, polysystemic vegetative symptoms, and the presence of emotional disorders, the severity of which can range from “a feeling of discomfort” to “panic” [11, 18]. The dominance of vegetative-somatic complaints that arise both at the time of PA and in the interictal period determines the predominance of such patients in the clinical practice of a neurologist, often causing diagnostic and therapeutic difficulties [1, 2, 8, 9].
Typical PA includes the symptoms listed above, and the fear of death almost obligately arises during the first attacks, and often accompanies all subsequent ones. However, in neurological and therapeutic practice, the clinical picture of paroxysms may differ significantly from the typical one. So, during an attack the patient may not experience fear and anxiety; such PAs are called “panic without panic” or “non-insurance PAs” [6, 9].
In the interictal period, patients, as a rule, develop permanent psycho-vegetative disorders, the structure of which is largely determined by the nature of the paroxysm. In patients with typical PA, soon after the onset of paroxysms, the so-called agoraphobic syndrome, literally – fear of open spaces (from agora – market square). In a broader sense, “agoraphobia” is defined as fear and/or avoidance of places or situations that may be difficult to escape or in which help cannot be provided if PA occurs. In patients with PA, fear concerns any situation that potentially “threatens” the development of an attack, which leads to the formation of restrictive behavior. A significant decrease in social activity due to illness is caused by the so-called. social demoralization, which leads to the development of secondary depression. In turn, depression is manifested by a decrease in food and sexual motivation, dyssomnia, severe weakness and fatigue [6, 9].
Over the years, leading medical institutions in Moscow conducted studies of the effectiveness of citalopram in neurological patients.
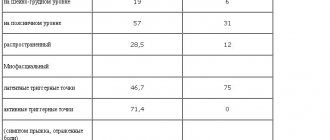
On the basis of the Department of Pathology of the Autonomic Nervous System of the Scientific Research Center of the First Moscow State Medical University named after. THEM. Sechenov conducted a dissertation study of the effectiveness of treatment of PA with citalopram (2001–2004). The study included 44 patients with PA – agoraphobia; the average age of the patients was 31.5 years [14]. The drug was prescribed at a dose of 20 mg/day for 8 weeks; none of the patients exceeded the indicated dose. The effectiveness of citalopram was assessed based on changes in psychometric testing indicators (levels of depression [3, 20] and anxiety [3, 20], panic attack typicality index [6], alexithymia scale [21], concentration and stability of attention, selectivity of attention according to the Munsterberg method ) and electrophysiological parameters. Of the 44 patients, 31 completed the full course of treatment with citalopram, 11 patients interrupted treatment for reasons unrelated to the side effects of the drug, 2 patients refused to take it due to dyspeptic disorders and a high degree of agitation in the first days of therapy. Five patients reported increased anxiety during the first two weeks of taking citalopram, which required diazepam 5 mg/day. The effectiveness of the therapy was 80.7%, which was characterized by a decrease in the frequency of PA by 30% or more, significant changes in all clinical and electrophysiological parameters, which were observed no earlier than 5-6 weeks from the start of therapy. Predictors of the effectiveness of treatment with citalopram were the typical clinical picture of a PA attack, shorter duration of the disease, fewer symptoms of vegetative accompaniment, low levels of anxiety, depression and alexithymia, and a higher level of attention. A significant advantage of citalopram was its positive effect on cognitive function.
On the basis of the Department of Pathology of the Autonomic Nervous System of the Scientific Research Center of the First Moscow State Medical University named after. THEM. Sechenov’s dissertation research (2000–2002) analyzed the effectiveness of citalopram in the treatment of panic disorders [5]. The study involved 44 patients suffering from panic disorder with or without agoraphobia in accordance with ICD-10 criteria [11], the average age of the patients was 29.9 years. The dose of citalopram did not exceed 20 mg/day, the duration of therapy was 8 weeks. Treatment effectiveness was assessed using the same methods as in the study [14]. The effectiveness of citalopram in 81.3% of cases was assessed as “high,” which was characterized by a reduction in PA and accompanying symptoms from 50% to complete disappearance; in 12% – as “average”, which was characterized by a reduction of PA and accompanying symptoms to less than 50%; in 6% – as “ineffective treatment”, when the patient’s well-being did not improve and symptoms persisted. When comparing groups of PA patients with and without agoraphobia, no significant differences were noted. The following factors were identified as negative predictors of effectiveness: the onset of the disease with anxiety, depression, full-blown attacks of PA with a predominance of vegetative symptoms, a high level of anxiety, a high degree of social maladjustment. Thus, 8-week therapy with citalopram demonstrated high effectiveness, independent of gender, as a factor in agoraphobia.
The types of depression in neurological patients are very diverse. Often, when visiting a neurologist, we encounter patients with reactive or psychogenic depression, which is a consequence of exposure to a traumatic situation, a response to acute or chronic stress, or a situation-related affective disorder.
Using a random sampling method, 20 patients (average age - 39.6 years) were examined who contacted a neurologist at the consultative and diagnostic department of MONIKI and were hospitalized in the neurological department of MONIKI [4]. Among the patients, there predominated people with situationally determined affective disorders who had a history of psycho-emotional disorders of varying severity and duration (from four weeks to three or more months). Pram (citalopram) was prescribed at a dosage of 10 mg/day during the 1st week of treatment, followed by an increase in dose to 20 mg/day. The observation period was 4 weeks. Affective disorders were assessed using the Hospital Anxiety and Depression Scale [3, 19]. Testing took place in 3 stages: before the start of therapy, after the 1st and 4th weeks of treatment. At baseline, severe anxiety (average score –15.6) and subclinically expressed depression (average score –9.4) were detected. The results obtained indicate the severity of predominantly anxiety disorders. During therapy with Pram, a significant decrease in the level of anxiety was observed, which was noted already in the 1st week of treatment at a dose of 10 mg/day, provided that Pram was prescribed as early as possible from the moment of the onset of the affective episode. The study showed the high clinical efficacy and safety of the drug Pram; only in one case did the patient experience clinically significant side effects that required discontinuation of treatment.
For a neurologist, the analgesic effect of antidepressants is very important. Currently, the priority in the treatment of chronic pain is antidepressants from the SSRI group, which have serotonergic activity. Their effectiveness in the treatment of chronic pain syndromes reaches 75%. The effectiveness of an antidepressant is higher, the greater the role depression plays in chronic pain [7].
The therapeutic efficacy of citalopram was assessed in the treatment of 18 patients with episodic (ETH) and chronic tension-type headaches (CTTH) [13]. Group with EGTH – 12 patients, average age – 25.1 years; group with CHF – 6 patients, average age – 38.6 years. The duration of the study was 56 days, the drug was prescribed as monotherapy, once in the morning, at a dose of 20 mg, 7 days after discontinuation of previous treatment (if any). The dynamics of the patients' condition was assessed by the following methods: clinical neurological examination, headache self-monitoring diaries, a scored questionnaire of autonomic dysfunction, a hyperventilation questionnaire [6]; assessment of the level of depression [3, 19] and anxiety [20]; neuropsychological testing – scale of alexithymia [21], concentration, stability and selectivity of attention. According to the headache diaries, the duration of the attack was 6 hours for EGTH and 12 hours for CGTH. The incidence of EGTH is 8 times a month, CGTH is almost every day. Both groups of patients had a moderately high level of depression, a high level of reactive and personal anxiety, the same intensity of headache on the visual analogue scale, mild alexithymia, and a mild impairment of stability and selectivity of attention. As a result of the treatment, in 77.8% of cases the drug was effective: the frequency of headache attacks decreased and its intensity decreased; in 22.2% of cases, treatment was ineffective: patients did not note a significant change in their condition. In the group with a positive effect, patients indicated an improvement in their general well-being: decreased asthenia, improved sleep quality, and a decrease in the severity of autonomic and hyperventilation disorders. The decrease in complaints of headaches was accompanied by a significant decrease in anxiety and depressive indicators and an improvement in the results of neuropsychological testing. Thus, citalopram is well tolerated, has a minimal number of side effects, and is highly effective in the treatment of patients with EGTH and CGTH, which are accompanied by anxiety-depressive and autonomic disorders.
Citalopram, along with other antidepressants (amitriptyline, imipramine, fluoxetine, mirtazapine, etc.) is included in the standard of treatment for patients with Parkinson's disease in the presence of depression without severe cognitive impairment (patient model - 7.1, stage - early, phase - initial treatment, without complications ), at a dosage of 20–40 mg/day. In the same dosages, citalopram is prescribed both in advanced (patient model - 7.2, phase - stable response to antiparkinsonian drugs) and late (patient model - 7.3, phase - unstable response to antiparkinsonian drugs, complications from long-term therapy; patient model - 7.4, phase – progressive, complications – impaired cognitive functions) stages of Parkinson’s disease in patients with severe depressive symptoms [12]. The use of SSRI drugs in patients with parkinsonism also has a beneficial effect on cognitive function.
Antidepressants are often necessary for a neurologist to achieve a good clinical effect of therapy. To prevent serious complications, a doctor should never combine two antidepressants with each other, as well as with other serotonin-mimetic drugs: fenfluramine, lithium salts. You should also know that a neurologist can treat with minimal and medium therapeutic doses and should never undertake the treatment of severe depressive conditions. This is the exclusive prerogative of psychiatrists [7].
Thus, in a number of studies, citalopram preparations, including Pram, have shown high effectiveness in the treatment of neurological patients suffering from panic attacks, situationally caused emotional disorders, and chronic pain syndromes. Pram is included in the standard treatment of depression in Parkinson's disease and is the drug of choice for the treatment of mild to moderate depression in epilepsy.
Information about the author: Shavlovskaya Olga Aleksandrovna – Candidate of Medical Sciences, leading researcher in the Department of Pathology of the Autonomic Nervous System of the National Research Center of the State Educational Institution of Higher Professional Education “First Moscow State Medical University named after. THEM. Sechenov” of the Ministry of Health and Social Development of the Russian Federation. Tel.
Side effects and other risks
Mechanism of side effects
Side effects are caused by an increase in serotonin. Most side effects occur immediately after starting treatment and go away over time.
Side effects
- Gastroenterological (reduced appetite, nausea, diarrhea, constipation)
- Insomnia, sedation, agitation, tremor
- Sweating
- Urinary dysfunction
- Dry mouth
- Dangerous side effects: seizures, mania, suicidal ideation
- Weight gain: very rare
- Sedation: yes, but infrequently
- Sexual dysfunction: yes
What to do about side effects
- Wait;
- For insomnia, take the drug in the morning;
- For sedation, take at night;
- Switch to another antidepressant [1].
Long term use
Safely
addictive
No.
Overdose
Very rare cases of fatal overdoses.
Vomiting, sedation, cardiac arrhythmia, dizziness, tremor