Glutamic acid is one of twenty amino acids essential for the body. Participates in nitrogen metabolism, binds ammonia and other substances toxic to the body. It is present in various foods and is part of medicines. Its analogue, made from plant materials, is included in some finished products as flavorings and spices.
When it comes to glutamic acid and the substances produced from it: monosodium, potassium, calcium, ammonium and magnesium glutamate, many are confused. According to some information, glutamate is harmless. Others classify it as a substance that can harm our body and deprive us of our natural sense of taste. What kind of substance is this, actually? Let's figure it out.
Pharmacodynamics and pharmacokinetics
Pharmacodynamics
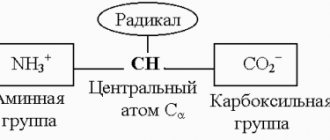
The Pharmacopoeia indicates that this drug improves the metabolism of cells of the nervous system. The structural formula of glutamic acid is C5H9NO4. A non-essential amino acid, present in the body only in the levorotatory form ( L glutamic acid a ). Acts as a mediator with pronounced metabolic activity in brain tissue, activates redox reactions in the brain, as well as protein metabolism. Regulates metabolism, transforming the functional status of the endocrine and nervous systems. Stimulates the transfer of excitation in neuronal synapses, promotes the neutralization and evacuation of ammonia , and increases resistance to hypoxia.
An important component of myofibrils , a component of the synthesis of other amino acids, ATP, acetylcholine, urea , helps transport and maintain the required content of potassium in brain tissue, serves as an intermediary between the metabolism of nucleic acids and carbohydrates, and normalizes the level of glycolysis in tissues. Has a hepatoprotective effect, suppresses the secretory function of stomach cells.
Pharmacokinetics
Has a high degree of absorption. It overcomes histohematic barriers, membranes of subcellular structures and cell membranes well. Accumulates in the liver, kidneys and soft tissues. Excreted in urine (5-7%) in its original form.
Glutamic acid (glutamate)
CAS number:
56-86-0
Gross formula:
C5H9NO4
Appearance:
white powder
Chemical name and synonyms:
L-Glutamic acid, L(+)-Glutamic acid;
2-Aminoglutaric acid Physico-chemical properties:
Molecular weight: 147.13 g/mol Density 1.538 Melting point 205 ºC alpha 32 º (c = 10.2 N HCl) Solubility in water 7.5 g/l (20 ºC). Very poorly soluble in cold water. Hazardous decomposition products formed under combustion conditions are carbon oxides, nitrogen oxides (NOx). The substance is stable under recommended storage conditions. Store in a tightly closed container in a cool, dry, ventilated area. Protect from physical damage. Protect from freezing.
Description:
Glutamic acid (or glutamic acid salt - glutamate) is a conditionally essential aliphatic (not having aromatic bonds in the structure) amino acid. Glutamate is used by the body to create proteins. It is the most abundant excitatory (stimulating) neurotransmitter in the central nervous system, and is also a metabolic intermediate in the Krebs cycle and a compound that may be involved in eliminating toxic ammonia from the body. When glutamic acid combines with ammonia, metabolic waste products are converted into glutamine. Glutamic acid plays a crucial role in maintaining a balanced acid-base ratio.
Glutamic acid is present in the body in fairly large quantities (up to 25%) as part of proteins and various chemicals, as well as in an unbound free state. It can be synthesized from oxoglutaric acid, produced during carbohydrate metabolism, and is biosynthesized from a number of amino acids, including ornithine and arginine. In a normal healthy body, glutamic acid is produced in sufficient quantities, but with age and in the presence of various pathologies in a person, its level may decrease. In this case, additional intake of glutamic acid is necessary, possibly in the form of dietary supplements.
Glutamic acid is also a precursor to GABA, an important neurotransmitter in the central nervous system. It helps transport potassium into the cerebrospinal fluid and is itself an excitatory neurotransmitter.
Sources of glutamic acid include soy, meat, poultry, fish, eggs and dairy products (especially cheese). But there is also a lot of it in plant foods: green peas, beets, corn, carrots, onions, spinach, etc.
Application:
Salt monosodium glutamate (MSG) is a common food additive and flavor enhancer that is widely used in the food industry. Glutamic acid is often used as a component in the production of sports nutrition and dietary supplements. It is also used in medicine for disorders of the nervous system. For example, for the treatment of mental retardation, epilepsy, Parkinson's disease, muscular dystrophy and alcoholism. Oral glutamine is used to reduce complications associated with sickle cell disease and short bowel syndrome in patients receiving nutritional support in combination with recombinant human growth hormone. Glutamine is also used for depression, irritability, anxiety, insomnia, diarrhea, Crohn's disease, cystic fibrosis, increased physical activity and recovery from burns. It is used orally for HIV depletion, leaky gut in people with HIV, mucositis caused by chemotherapy, chemotherapy or antiretroviral diarrhea, neuropathy or lymphocytopenia caused by chemotherapy, diabetic foot ulcers, bedsores, and to protect the immune system and intestines. Glutamic acid has a barrier function in people with esophageal cancer undergoing radiochemotherapy. It is also used for weight loss, attention deficit hyperactivity disorder (ADHD), cystinuria, peptic ulcers, ulcerative colitis, pancreatitis, muscular dystrophy, bone marrow transplant recovery, and to support opiate or alcohol withdrawal. Glutamic acid is administered orally as an enteral nutrition to prevent morbidity in trauma patients, prevent infectious complications in critically ill patients, and to treat paclitaxel-induced myalgia and arthralgia. In premature or low birth weight infants, glutamine is used to reduce morbidity and mortality.
L-glutamic acid is also used in cell culture as a component of the MEM solution of essential amino acids. L-glutamic acid was used as a nitrogen source in Aspergillus fumigatus NRRL 2436 for the production of fumagillin.
Receipt:
The method of obtaining L-glutamic acid by biochemical means involves the use of pyrrolidone carboxylic acid as a raw material. Pyrrolidone carboxylic acid (hereinafter abbreviated as PCA) is obtained through organic synthesis, extraction from natural materials, intramolecular dehydration reaction of glutamic acid, and so on. L-glutamic acid, which is obtained by hydrolytic transformation of PCA, has been known to be commercially useful. However, according to the conventional method, L-PCA is converted to L-glutamic acid, and D-PCA is converted to D-glutamic acid, and DL-PCA is converted to DL-glutamic acid, respectively. To obtain L-glutamic acid from D-PCA or DL-PCA, another process such as optical resolution or racemization is required besides the conversion process. And this is achieved by contacting D-PCA and/or DL-PCA with a microorganism as an enzymatic agent in an aqueous medium having a pH range of -10 in the presence of air and in a temperature range of 25-60 C. Both hydrolysis and optical racemization reactions are hypothesized to occur simultaneously during this biochemical reaction in which L-glutamic acid is derived from D-PCA and/or DL-PCA. Although the detailed mechanism is not yet understood scientifically, it is a fact that only L-glutamic acid is formed without any trace of D-glutamic acid. In addition, L-glutamic acid is, of course, easily formed from L-PCA when it exists in the reaction system. Most of the microorganisms used in this method are strains belonging to the family Achromobacteriaceae, Micrococcaceae, Brevibacteriaceae, Corynebacteriaceae, Enterobacteriaceae, Pseudomonadaceae, Bacillaceae, Rhizobiceae, and can be easily obtained from nature and culture. However, any microorganism that can metabolize D-PCA into L-glutamic acid can be used, even if it does not belong to one of these families. (This method belongs to researchers Yoshio Kawai, Zushi-shi, Teijiro Uernura and Yasuo Kawai, Tokyo, and Shinji Olrumura, Yokohama, Japan, assignors to Sanko Co., Inc., Kanagawa-lren, Japan, a corporation or Japan No Drawing) .
Effect on the body:
L-glutamic acid (L-GA) exists physiologically as glutamate. Glutamate plays an important role in the metabolism of amino acids and therefore in maintaining nitrogen balance in the body. It is a well-known excitatory neurotransmitter in the central nervous system. There is compelling evidence for the protective activity of L-GA and α-ketoglutarate in vincristine-induced neurotoxicity. The possible immunomodulatory role of L-glutamine can be explained in several ways. L-glutamine appears to play a major role in protecting the integrity of the gastrointestinal tract and the colon in particular. During catabolic states, the integrity of the intestinal mucosa may be compromised, with subsequent increased intestinal permeability and movement of gram-negative bacteria from the colon into the body. The requirement for L-glutamine in the intestine, as well as in cells such as lymphocytes, appears to be much higher than in skeletal muscle, the main storage tissue for L-glutamine. L-glutamine is the preferred fuel for enterocytes, colonocytes and lymphocytes. Therefore, supplementing with L-glutamine may solve some problems. On the one hand, it can reverse the catabolic state by sparing skeletal muscle L-glutamine. This will also prevent the translocation of gram-negative bacteria from the colon. L-glutamine helps maintain secretory IgA, which functions primarily by preventing bacteria from attaching to mucosal cells. L-glutamine appears to be required to support the proliferation of mitogen-stimulated lymphocytes as well as the production of interleukin-2 (IL-2) and interferon-gamma (IFN-gamma). It is important for the maintenance of lymphokine-activated killer cells (LACs). L-glutamine may enhance phagocytosis of neutrophils and monocytes. This may lead to increased glutathione synthesis in the intestine, which may also play a role in maintaining the integrity of the intestinal mucosa by reducing oxidative stress. The exact mechanism of the possible immunomodulatory effect of supplemental L-glutamine, however, remains unclear. It is possible that the main effect of L-glutamine occurs in the intestines. It is possible that enteral L-glutamine acts directly on gut-associated lymphoid tissue and stimulates overall immune function through this mechanism.
Toxicological data:
Acute toxicity. LD50 for oral administration - rat -> 30,000 mg/kg.
Indications for use
Indications for the use of Glutamic acid (in complex therapy):
- inhibition of mental development of various etiologies, cerebral palsy , consequences after birth intracranial trauma, poliomyelitis , Down's disease ;
- schizophrenia, epilepsy (minor seizures), psychosis , depressive reactive state, insomnia , mental exhaustion , consequences of encephalitis and meningitis , progressive myopathy , depression ;
- neuropathy of toxic origin due to the use of isonicotinic acid derivatives.
special instructions
If the treatment is in powder form, it is necessary to rinse the mouth after each dose with a weak solution of sodium bicarbonate to reduce the acidity of the environment and protect the teeth from destruction.
Glutamic acid can be used as a treatment for various neurotoxic reactions that occur while taking other medications.
Taking the drug by children of preschool and school age should be accompanied by an accurate calculation of a single dose to avoid a possible overdose.
Glutamic acid, instructions for use (Method and dosage)
The medicine is taken orally half an hour before meals; if symptoms of dyspepsia it is used after or during meals.
Adult patients are prescribed 1 gram of the drug up to three times a day.
- Children under 1 year of age are prescribed 100 mg per day.
- Up to 2 years of age, 150 mg per day is prescribed.
- Children 3-4 years old are prescribed 250 mg per day.
- Children 5-6 years old are prescribed 400 mg per day.
- Children 7-9 years old are prescribed 500-1000 mg per day.
- Children over 10 years of age are prescribed 1000 mg up to three times a day.
For oligophrenia, the drug is prescribed at the rate of 100-200 mg per kilogram of weight.
The duration of treatment is usually from 1-2 months to 1 year.
Properties
| Index | Standard values |
| Color | white |
| Compound | glutamic acid, empirical formula C5H9NO4 |
| Appearance | crystalline powder, granules, crystals |
| Smell | absent |
| Solubility | good in hot water, alkaline solutions, dilute acids; difficult in cold water; practically insoluble in alcohols, ethers |
| Main substance content | 99–101% |
| Taste | soft, with a metallic aftertaste |
| Density | 1.46 g/cm3 |
| Other | melting point 160ºC; resistant to hydrolysis by acids and alkalis; has low hygroscopicity |
Analogs
Level 4 ATX code matches:
Bravinton
Acefen
Carnicetine
Pyracesin
Nooclerin
Semax
Piracetam
Olatropil
Fezam
Vinpocetine
Cerebrocurin
Cavinton Forte
Calcium hopantenate
Cephabol
Olanzapine
Cerebrolysate
Pramistar
Sidnocarb
Vinpotropil
Glycine Ozone
Antifront, Armadin, Glycine, Instenon, Intellan, Cortexin, Neurotropin, Rilutek, Cytoflavin.
Reviews about Glutamic acid
Reviews of Glutamic acid show that the drug is used only in combination with more potent drugs and is more similar in action to dietary supplements. The evidence base for the benefits of the drug for this or that pathology is weak. Glutamic acid is used separately in sports and bodybuilding.
Glutamic acid in bodybuilding and sports
The drug is often included in the diet of athletes to increase performance. It helps to recover faster after intense physical exertion, as it reduces intoxication metabolic products and has a weak anabolic effect.
Possible adverse reactions
The following are observed as undesirable reactions in patients:
- sleep disorders;
- abdominal pain;
- frequent loose stools;
- increased nervous excitability;
- chills;
- leukopenia;
- cracks around the mouth and lips;
- short-term increase in body temperature.
Long-term treatment may cause a decrease in hemoglobin levels. In this regard, it is necessary to regularly check the condition of the body through a laboratory blood test.
Glutamic acid price, where to buy
Buying 10 tablets of the drug in Russia will cost approximately 28 rubles.
The price of Glutamic acid in a pharmacy in Ukraine (the same release form) is 5-6 hryvnia.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
Pharmacy Dialogue
- Glutamic acid Vitamir (table 500 mg No. 30) Square C
99 rub. order
show more
Pharmacy24
- Glutamic acid No. 10 tablets PAT "Kiev Vitamin Plant", Kiev, Ukraine
7 UAH. order
Type of substance
Additive E 620 belongs to the group of substances that enhance the taste and aroma of food products. It is a conditionally essential dicarboxylic amino acid of the aliphatic series.
Lutamic acid can be obtained in various ways, including chemical synthesis. Hence, consumers have a widespread belief that the flavor enhancer E 620 is an artificial product.
This is wrong. The chemical process of obtaining the additive is technically complex and requires serious material costs.
The most common other methods for producing glutamic acid L(+):
- by microbiological synthesis. To obtain the additive, gram-positive, non-spore-forming bacteria of the genera Micrococcus, Brevibacterium and Microbacterium are used;
- from α-ketoglutaric acid by enzymatic fermentation.
Both methods are cost-effective and environmentally friendly. The output is natural glutamic acid.
Interesting fact! There is another reason why the food industry does not use the synthetic additive E 620. Glutamic, like any amino acid, exists in two isomers: L- (from the Latin laevus, left) and D- (dexter, right). The first is found in nature and participates in all biochemical processes occurring in the body. It is so important that the human tongue has special receptors sensitive to glutamic acid L+. Thanks to them, we perceive the taste of protein products.
When producing the additive, a racemate of DL isomers is obtained artificially; it is difficult to separate them.
The D-isomer does not occur in nature; language receptors do not recognize it. In other words, it is completely useless as a flavor enhancer; there is no point in adding it to products.