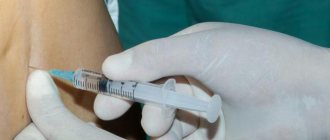
Indications for use
GCS should be used only as symptomatic treatment, with the exception of some endocrine disorders, for which they are used as replacement therapy. A. IM APPLICATION Methylprednisolone acetate (DEPO-MEDROL®) is not used for the treatment of acute life-threatening conditions. If a rapid hormonal effect of maximum intensity is required, then highly soluble methylprednisolone sodium succinate (SOLU-MEDROL®) is prescribed intravenously. If it is not possible to carry out oral therapy with GCS, then intramuscular use of the drug is indicated for the following diseases:
Endocrine diseases:
- primary and secondary adrenal insufficiency (drugs of choice - hydrocortisone or cortisone; if necessary, in combination with mineralocorticoids, especially in pediatric practice);
- acute adrenal insufficiency (drugs of choice are hydrocortisone or cortisone; it may be necessary to add mineralocorticoids);
- congenital adrenal hyperplasia;
- hypercalcemia due to cancer;
- subacute thyroiditis.
Rheumatic diseases:
As an additional agent for maintenance therapy (non-steroidal anti-inflammatory drugs, kinesiotherapy, physiotherapy, etc.) and for short-term use (to remove the patient from an acute condition or during an exacerbation of the process) for the following diseases:
- psoriatic arthritis;
- ankylosing spondylitis.
For the following diseases, the drug should be used in situ whenever possible:
- post-traumatic osteoarthritis;
- synovitis in osteoarthritis;
- rheumatoid arthritis, including juvenile rheumatoid arthritis (in some cases, low-dose maintenance therapy may be required);
- acute and subacute bursitis;
- epicondylitis;
- acute nonspecific tenosynovitis;
- acute gouty arthritis.
Collagenoses: During the period of exacerbation or in some cases as maintenance therapy for the following diseases:
- systemic lupus erythematosus;
- systemic dermatomyositis (polymyositis);
- acute rheumatic myocarditis.
Skin diseases:
- pemphigus;
- malignant exudative erythema (Stevens-Johnson syndrome);
- exfoliative dermatitis;
- mycosis fungoides;
- bullous dermatitis herpetiformis (the drug of choice is sulfone, systemic use of GCS is adjuvant).
Allergic Conditions: For the control of the following severe and disabling allergic conditions that cannot be treated by conventional methods:
- status asthmaticus;
- contact dermatitis;
- atopic dermatitis;
- serum sickness;
- seasonal or year-round allergic rhinitis;
- drug allergies;
- reactions to transfusions and administration of drugs such as urticaria;
- acute non-infectious laryngeal edema (drug of choice - epinephrine).
Ophthalmological diseases: Severe acute and chronic allergic and inflammatory processes with eye damage, such as:
- uveitis and inflammatory eye diseases that do not respond to topical corticosteroids.
Diseases of the gastrointestinal tract: To remove a patient from a critical condition with the following diseases:
- ulcerative colitis (systemic therapy);
- Crohn's disease (systemic therapy).
Respiratory diseases:
- symptomatic sarcoidosis;
- berylliosis;
- focal or disseminated pulmonary tuberculosis (used in combination with appropriate anti-tuberculosis chemotherapy);
- Loeffler's syndrome, which cannot be treated with other methods;
- aspiration pneumonitis.
Hematological diseases:
- acquired (autoimmune) hemolytic anemia;
- secondary thrombocytopenia in adults;
- erythroblastopenia (thalassemia major);
- congenital (erythroid) hypoplastic anemia.
Oncological diseases: As palliative therapy for the following diseases:
- leukemia and lymphoma in adults.
Edema syndrome: For induction of diuresis or treatment of proteinuria in nephrotic syndrome, idiopathic type or due to systemic lupus erythematosus Nervous system:
- multiple sclerosis in the acute phase.
Other indications for use:
- tuberculous meningitis with subarachnoid block or threatened block, in combination with appropriate anti-tuberculosis chemotherapy.
- trichinosis with damage to the nervous system or myocardium.
INTRA-ARTICULAR, PERIARTICULAR, INTRABURSAL APPLICATION AND INTRODUCTION TO SOFT TISSUE. As an adjuvant therapy for short-term use (to remove a patient from an acute condition or during an exacerbation of the process) for the following diseases:
- synovitis in osteoarthritis;
- rheumatoid arthritis;
- acute and subacute bursitis;
- acute gouty arthritis;
- eicondylitis;
- acute nonspecific tenosynovitis.
INTRODUCTION TO PATHOLOGICAL FOCUS: Keloid scars and localized foci of inflammation with:
- lichen planus (Wilson's);
- psoriatic plaques;
- granuloma annulare;
- simple chronic lichen (neurodermatitis);
- discoid lupus erythematosus;
- alopecia areata.
Also effective for cystic tumors or tendon aponeurosis (tendon sheath cysts).
Interactions of the drug Depo-Medrol
With the simultaneous use of methylprednisolone and cyclosporine, a mutual decrease in the intensity of their metabolism is observed. Therefore, when these drugs are used together, the likelihood of adverse reactions that may occur when either drug is used as monotherapy increases. There have been cases of seizures with simultaneous use of methylprednisolone and cyclosporine. The simultaneous administration of inducers of microsomal liver enzymes such as barbiturates, phenytoin and rifampicin can increase the metabolism of GCS and weaken the effectiveness of GCS therapy. In this regard, it may be necessary to increase the dose of Depo-Medrol to obtain the required therapeutic effect. Drugs such as oleandomycin and ketoconazole can inhibit the metabolism of corticosteroids, so dose selection of the corticosteroid should be done with caution to avoid overdose. GCS may increase the renal clearance of salicylates. This may lead to a decrease in serum salicylate levels and to salicylate toxicity if the administration of corticosteroids is discontinued. In case of hypoprothrombinemia, acetylsalicylic acid in combination with GCS should be used with caution. GCS can both weaken and enhance the effect of anticoagulants. In this regard, anticoagulant therapy should be carried out under constant monitoring of blood coagulation parameters. In the treatment of fulminant and disseminated pulmonary tuberculosis and tuberculous meningitis with subarachnoid block or threatened block, methylprednisolone is administered simultaneously with appropriate anti-tuberculosis chemotherapy. GCS may increase the need for insulin and oral hypoglycemic drugs in patients with diabetes mellitus. The combination of GCS with thiazide diuretics increases the risk of decreased glucose tolerance. The simultaneous use of drugs that have an ulcerogenic effect (for example, salicylates and other NSAIDs) may increase the risk of ulceration in the gastrointestinal tract.
Directions for use and doses
- i/m;
- intra-articular, periarticular, intrabursal or soft tissue injection;
- introduction into the pathological focus.
INTRODUCTION TO THE PATHOLOGICAL FOCUS TO ACHIEVE A LOCAL EFFECT. Despite the fact that treatment with DEPO-MEDROL® leads to a reduction in the symptoms of the disease, it does not affect the cause of the inflammatory process, therefore it is necessary to carry out the usual therapy for each specific disease. Rheumatoid arthritis and osteoarthritis. The dose for intra-articular administration depends on the size of the joint, as well as the severity of the patient’s condition. For chronic conditions, the number of injections may vary from one to five or more per week, depending on the degree of improvement achieved after the first injection. The following dosages are given as general recommendations: Joint size Joint name Dose range Large Knee Ankle Shoulder 20-80 mg Medium Elbow Wrist 10-40 mg Small Metacarpophalangeal Interphalangeal 4-10 mg Procedure. Before performing an intra-articular injection, it is recommended to evaluate the anatomy of the affected joint. For a full anti-inflammatory effect, it is important that the injection is carried out into the synovial cavity. It is necessary to follow the rules of asepsis and antisepsis in the same way as during lumbar puncture. A sterile 20-24 G needle (attached to a dry syringe) is quickly inserted into the synovial cavity. The method of choice is infiltration anesthesia with procaine. To control the entry of the needle into the joint cavity, a few drops of intra-articular fluid are aspirated. When choosing the injection site, which is individual for each joint, the proximity of the synovial cavity to the surface (as close as possible), as well as the passage of large vessels and nerves (as far as possible), is taken into account. The needle remains in place, the syringe with the aspirated liquid is removed and replaced with another syringe containing the required amount of DEPO-MEDROL®. You should then slowly pull the plunger toward you and aspirate the synovial fluid to ensure that the needle is still in the synovial cavity. After the injection, you should make a few light movements in the joint, which helps mix the suspension with the synovial fluid. The injection site is covered with a small sterile bandage. The drug can be injected into the knee, ankle, elbow, shoulder, metacarpophalangeal, interphalangeal and hip joints. Sometimes there are difficulties with insertion into the hip joint, since large blood vessels must be avoided. Injections are not made into the following joints: anatomically inaccessible joints, for example, intervertebral joints, including the sacroiliac joint, which lacks a synovial cavity. The failure of therapy is most often the result of an unsuccessful attempt to penetrate the joint cavity. When the drug is introduced into surrounding tissues, the effect is insignificant or absent altogether. If therapy does not produce positive results in cases where entry into the synovial cavity is beyond doubt, as confirmed by aspiration of intra-articular fluid, repeated injections are usually useless. Local therapy does not affect the process underlying the disease, so complex therapy should be carried out, including basic anti-inflammatory therapy, physiotherapy and orthopedic correction. After intra-articular administration of GCS, care should be taken not to overload joints in which symptomatic improvement has been noted in order to avoid more severe damage to the joint compared to what was before the start of GCS therapy. GCS should not be injected into unstable joints. In some cases, repeated intra-articular injections can lead to joint instability. In some cases, it is recommended to carry out x-ray control to identify damage. If a local anesthetic is used before administering DEPO-MEDROL®, you should carefully read the instructions for use of this anesthetic to ensure all necessary precautions are taken. Bursitis. After treating the area around the injection site with a suitable antiseptic, local infiltration anesthesia is performed with a 1% procaine solution. A 20-24 G needle is placed on a dry syringe, which is inserted into the joint capsule, and then the liquid is aspirated. The needle is left in place, and the syringe with the aspirated liquid is removed and a syringe containing the required dose of the drug is installed in its place. After the injection, the needle is removed and a bandage is applied. Tendon sheath cyst, tendonitis, epicondylitis. When treating conditions such as tendinitis or tenosynovitis, care must be taken to ensure that the suspension is injected into the tendon sheath and not into the tendon tissue. The tendon can be easily palpated by running your hand along it. When treating conditions such as epicondylitis, the most painful area should be identified and the suspension should be injected into it using the creeping infiltration method. For tendon sheath cysts, the suspension is injected directly into the cyst. In many cases, it is possible to achieve a significant reduction in the size of the cystic tumor and even its disappearance after a single injection of the drug. Each injection should be done in compliance with the rules of asepsis and antiseptics (treating the skin with a suitable antiseptic). The dose is selected depending on the nature of the process and is 4-30 mg. In case of relapses or chronic course of the process, repeated injections may be required. Skin diseases. After treating the skin with a suitable antiseptic, for example, 70% alcohol, 20-60 mg of the suspension is injected into the lesion. For a large affected area, a dose of 20-40 mg is divided into several parts and injected into different areas of the affected surface. When administering the drug, care should be taken to avoid whitening of the skin, which may subsequently lead to peeling. Usually 1-4 injections are performed, the interval between injections depends on the type of pathological process and on the duration of the period of clinical improvement achieved after the first injection. IM ADMINISTRATION TO ACHIEVE A SYSTEMIC EFFECT. The dose of the drug for IM administration depends on the disease being treated. To obtain a long-term effect, calculate the weekly dose by multiplying the daily oral dose by 7, and administer it as one intramuscular injection. The dose should be selected individually, taking into account the severity of the disease and the patient's response to therapy. In children (including infants), a lower dose is used, which is chosen primarily based on the severity of the disease, rather than using constant regimens calculated on the basis of age or body weight. The course of treatment should be as short as possible. Treatment is carried out under constant medical supervision. Hormone therapy is an addition to conventional therapy, but does not replace it. The dose of the drug should be reduced gradually, and the drug should also be discontinued gradually if it was administered for longer than several days. The main factors determining the choice of dose are the severity of the disease, prognosis, expected duration of the disease, and the patient's response to therapy. If a period of spontaneous remission occurs in a chronic disease, treatment should be interrupted. During long-term therapy, routine laboratory tests, such as a general urine test, determination of blood glucose concentration 2 hours after a meal, determination of blood pressure, body weight, and chest x-ray should be performed regularly at certain intervals. In patients with a history of gastric and duodenal ulcers or severe dyspepsia, it is advisable to undergo an X-ray examination of the upper gastrointestinal tract. For patients with adrenogenital syndrome, it is enough to administer 40 mg intramuscularly once every 2 weeks. For maintenance therapy in patients with rheumatoid arthritis, the drug is administered intramuscularly at a dose of 40-120 mg once a week. The usual dose for systemic GCS therapy in patients with skin diseases, which allows achieving a good clinical effect, is 40-120 mg IM once a week for 1-4 weeks. In acute severe dermatitis caused by the poison contained in ivy, it is possible to eliminate the symptoms within 8-12 hours after a single intramuscular injection of 80-120 mg. For chronic contact dermatitis, repeated injections at intervals of 5-10 days may be effective. For seborrheic dermatitis, to control the condition, it is enough to administer 80 mg once a week. After intramuscular administration of 80-120 mg to patients with bronchial asthma, symptoms disappear within 6-48 hours, and the effect persists for several days or even 2 weeks. In patients with allergic rhinitis (hay fever), an intramuscular injection of 80-120 mg can also eliminate the symptoms of acute rhinitis within 6 hours, with the effect lasting from several days to 3 weeks. If the disease for which therapy is aimed also develops symptoms of stress, the dose of the suspension should be increased. To obtain a quick maximum effect, intravenous administration of methylprednisolone sodium succinate, characterized by rapid solubility, is indicated.
Pharmacological properties of the drug Depo-medrol
Depo-Medrol is a sterile aqueous suspension of the synthetic corticosteroid methylprednisolone acetate. It has a pronounced and long-lasting anti-inflammatory, antiallergic and immunosuppressive effect. Depo-Medrol is used intramuscularly to achieve a prolonged systemic effect, as well as in situ as a means for local (local) therapy. The prolonged effect of the drug is explained by the slow release of the active substance. Methylprednisolone acetate has the same properties as methylprednisolone, but is less soluble and less actively metabolized, which explains its longer duration of action. GCS penetrate cell membranes and form complexes with specific cytoplasmic receptors. Then these complexes penetrate the cell nucleus, bind to DNA (chromatin) and stimulate the transcription of mRNA and further synthesis of various enzymes, which explains the effect of systemic use of GCS. The latter not only have a pronounced effect on the inflammatory process and the immune response, but also affect carbohydrate, protein and fat metabolism, the cardiovascular system, skeletal muscles and the central nervous system. Most indications for the use of GCS are due to their anti-inflammatory, immunosuppressive and antiallergic properties. Thanks to these properties, the following therapeutic effects are achieved: reducing the number of immunoactive cells at the site of inflammation; decreased vasodilation; stabilization of lysosomal membranes; inhibition of phagocytosis; decreased production of prostaglandins and related compounds. At a dose of 4.4 mg, methylprednisolone acetate (4 mg methylprednisolone) exhibits the same anti-inflammatory effect as hydrocortisone at a dose of 20 mg. Methylprednisolone has minimal mineralocorticoid effects (200 mg methylprednisolone is equivalent to 1 mg deoxycorticosterone). GCS exhibit a catabolic effect on proteins. The amino acids that are released are converted by the process of gluconeogenesis in the liver into glucose and glycogen. Glucose consumption in peripheral tissues is reduced, which can lead to hyperglycemia and glycosuria, especially in patients who are prone to diabetes mellitus. GCS have a lipolytic effect, which primarily manifests itself in the extremities. GCS also exhibit lipogenetic effects, which are most pronounced in the chest, neck and head. All this leads to the redistribution of fat deposits. The maximum pharmacological activity of GCS occurs when maximum concentrations in the blood plasma decrease, so the effect of GCS is primarily due to their effect on enzyme activity. Methylprednisolone acetate is hydrolyzed by serum cholinesterases to form an active metabolite. In the human body, methylprednisolone forms a weak, dissociating bond with albumin and transcortin. Approximately 40–90% of the drug is bound. Due to the intracellular activity of GCS, there is a pronounced difference between the plasma half-life and the pharmacological half-life. Pharmacological activity persists even when the level of the drug in the blood is no longer determined. The duration of the anti-inflammatory effect of GCS is approximately equal to the duration of inhibition of the hypothalamic-pituitary-adrenal axis. After intramuscular administration of the drug at a dose of 40 mg/ml, the maximum concentration in the blood serum was reached on average after 7.3 ± 1 hour or averaged 1.48 ± 0.86 mcg/100 ml, the half-life was 69.3 hours After a single intramuscular injection of 40–80 mg of methylprednisolone acetate, the duration of inhibition of the hypothalamic-pituitary-adrenal axis was 4-8 days. After intra-articular injection of 40 mg into each knee joint (total dose - 80 mg), the maximum serum concentration was reached after 4-8 hours and was approximately 21.5 mcg/100 ml. The release of the drug into the systemic circulation from the joint cavity persisted for approximately 7 days, which is confirmed by the duration of inhibition of the hypothalamic-pituitary-adrenal axis and the results of determining the concentration of methylprednisolone in the blood serum. Metabolism of methylprednisolone occurs in the liver; this process is qualitatively similar to that for cortisol. The main metabolites are 20-β-hydroxymethylprednisolone and 20-β-hydroxy-6-α-methylprednisone. Metabolites are excreted in the urine in the form of glucuronides, sulfates and unconjugated compounds. Conjugation reactions occur mainly in the liver and partly in the kidneys.
Contraindications
- intrathecal administration;
- intravenous administration;
- systemic fungal infections;
- established hypersensitivity to any component of the drug.
With caution: with eye damage caused by the herpes simplex virus; as this may lead to corneal perforation; with ulcerative colitis, if there is a threat of perforation, development of an abscess or other purulent infection, as well as with diverticulitis; in the presence of fresh intestinal anastomoses; with active or latent peptic ulcer; renal failure; diabetes mellitus; arterial hypertension; osteoporosis; myasthenia gravis, when GCS are used as primary or additional therapy; with a history of mental disorders; in children.
special instructions
Use strictly as prescribed by your doctor to avoid complications.
- preparations for parenteral administration should be visually inspected before use to identify foreign particles and changes in the color of the drug;
- bottles must not be stored upside down! Shake well before use;
- one bottle cannot be used to administer multiple doses; after administering the required dose, the bottle with the remaining suspension should be destroyed;
- DEPO-MEDROL® should not be administered by any other route. Administration of the drug by any other route not approved by the developer is associated with serious adverse reactions, including: arachnoiditis, meningitis, paraparesis/paraplegia, sensory disturbances, bowel and bladder dysfunction, seizures, visual disturbances including blindness, eye inflammation and its appendages, residual effects or foci of rejection of necrotic tissue at the injection site;
- Since GCS crystals suppress inflammatory reactions, their presence can cause degradation of cellular and extracellular elements of connective tissue, which in rare cases manifests itself in the form of skin deformation at the injection site. The severity of these changes depends on the amount of GCS administered. After complete absorption of the drug (usually after several months), complete regeneration of the skin at the injection site occurs;
- To minimize the likelihood of developing skin or subcutaneous tissue atrophy, care should be taken not to exceed the recommended dose for parenteral administration. If possible, the affected area should be mentally divided into several areas and a portion of the total dose of the drug should be injected into each of them. When carrying out intra-articular and intramuscular injections, care must be taken not to inject the drug into the skin, or to prevent the drug from getting into the skin, and also not to accidentally inject the drug into the deltoid muscle, as this can lead to the development of atrophy of the subcutaneous tissue;
- if patients receiving GCS therapy may be or have already been exposed to severe stress, increased doses of fast-acting GCS should be administered before, during and after this exposure;
- GCS can erase the clinical picture of an infectious disease; with their use, new infections can develop. Against the background of GCS therapy, the body's resistance to infection may decrease, and the body's ability to localize the infectious process is also impaired. The development of infections caused by various pathogenic organisms, such as viruses, bacteria, fungi, protozoa or helminths, which are localized in various systems of the human body, may be associated with the use of GCS, both as monotherapy and in combination with other drugs - immunosuppressants , affecting cellular immunity, humoral immunity or neutrophil function. These infections may not be severe, but in some cases they can be severe and even fatal. Moreover, the higher the doses of GCS used, the higher the likelihood of developing infectious complications. In case of acute infection, the drug should not be administered intra-articularly, into the joint capsule or into the tendon sheaths of the muscles; IM administration is possible only after selecting appropriate antimicrobial/antiparasitic therapy;
- with long-term use of GCS, posterior subcapsular cataract and glaucoma with possible damage to the optic nerve may develop; the likelihood of developing secondary infections caused by fungi and viruses increases;
- in children receiving GCS therapy for a long time daily, growth retardation may occur. This mode of administration should be used only in the most severe conditions;
- For patients receiving treatment with corticosteroids in doses that have an immunosuppressive effect, the administration of live or live attenuated vaccines is contraindicated. However, killed or inactivated vaccines can be administered to patients receiving treatment with corticosteroids in doses that have an immunosuppressive effect; however, the response to such vaccines may be reduced. Patients receiving treatment with GCS in doses that do not have an immunosuppressive effect can be immunized according to appropriate indications;
- the use of DEPO-MEDROL® for active tuberculosis is indicated only in cases of focal or disseminated tuberculosis when GCS are used in combination with appropriate anti-tuberculosis chemotherapy. If GCS are prescribed to patients with latent tuberculosis or during the period of tuberculin testing, then the doses should be selected especially carefully, because reactivation of the disease may occur. During long-term GCS therapy, such patients should receive anti-tuberculosis chemoprophylaxis;
- Since patients receiving GCS therapy may, in rare cases, develop an anaphylactic reaction, appropriate precautions should be taken before administration, especially if the patient has a history of allergic reactions to any drug. The observed allergic skin reactions were apparently due to inactive components. In rare cases, skin tests have revealed reactions to methylprednisolone itself;
- During GCS therapy, the development of various mental disorders is possible: from euphoria, insomnia, mood swings, personality disorders and severe depression to acute psychotic manifestations.
WHEN PARENTERAL ADMINISTRATION OF GCS IT IS NECESSARY TO OBSERVE THE FOLLOWING ADDITIONAL PRECAUTIONS.
- with intra-articular administration of GCS, both systemic and local side effects may occur;
- it is necessary to conduct an appropriate study of the aspirated joint fluid to exclude a septic process;
- a significant increase in pain, accompanied by local swelling, further limitation of movements in the joint, fever and soreness are signs of septic arthritis. If such a complication develops and the diagnosis of sepsis is confirmed, local administration of GCS should be stopped and adequate antimicrobial therapy should be prescribed;
- GCS should not be injected into a joint that previously had an infectious process;
- GCS should not be injected into unstable joints;
- it is necessary to comply with the rules of asepsis and antiseptics to prevent infections and contamination;
- It should be taken into account that the absorption of methylprednisolone with intramuscular administration occurs more slowly;
- Although controlled clinical studies have shown that GCS effectively accelerate the recovery process during exacerbation of multiple sclerosis, it has not been established that GCS affect the outcome and pathogenesis of this disease. Studies have also shown that to achieve a significant effect, it is necessary to administer sufficiently high doses of GCS;
- since the severity of complications during the treatment of GCS depends on the dose and duration of therapy, in each specific case the potential risk and the expected positive effect should be compared when choosing the dose and duration of treatment, as well as when choosing between daily administration and intermittent administration;
- It is reported that Kaposi's sarcoma was observed in patients receiving GCS therapy. However, when GCS is discontinued, clinical remission may occur;
- there is no evidence that GCS have carcinogenic or mutagenic effects or affect reproductive function.
Effect on the ability to drive a car and operate machinery. Although visual impairment is rare when taking the drug, patients taking DEPO-MEDROL® should exercise caution when driving a car or operating other mechanisms.
Side effects of the drug Depo-Medrol
When treating GCS, including methylprednisolone, such side effects are possible. Water and electrolyte imbalance: sodium and fluid retention in the body, hypertension (arterial hypertension), congestive heart failure (in patients with risk factors), potassium loss, hypokalemic alkalosis. From the musculoskeletal system: steroid myopathy, muscle weakness, osteoporosis, pathological fractures, vertebral compression fractures, aseptic bone necrosis, tendon ruptures, especially the Achilles tendon. From the digestive tract: peptic ulcer of the digestive tract (including with bleeding and perforation), gastrointestinal bleeding, pancreatitis, esophagitis, intestinal perforation, transient and moderate increase in the activity of ALT, AST and alkaline phosphatase in the blood serum without any clinical manifestations (pass spontaneously after discontinuation of the drug). From the skin: delayed wound healing, petechiae, ecchymosis, thinning and fragility of the skin. From the side of metabolic processes: negative nitrogen balance due to protein catabolism. From the side of the central nervous system: increased intracranial pressure, pseudotumor cerebri, epileptic seizures. From the endocrine system: menstrual irregularities, Cushingoid syndrome, suppression of the pituitary-adrenal axis, decreased tolerance to carbohydrates, manifestation of latent diabetes mellitus, increased need for insulin or oral hypoglycemic drugs in patients with diabetes, slowed growth in children. From the organs of vision: posterior subcapsular cataract, increased intraocular pressure, exophthalmos. On the part of the immune system: erased clinical picture in infectious diseases, activation of latent infections caused by opportunistic pathogens, hypersensitivity reactions, including anaphylaxis, suppression of reactions during skin tests with allergens. With parenteral administration of GCS, the following side effects are possible: rarely - cases of blindness associated with local administration of the drug to pathological foci located in the face and head; allergic reactions (including anaphylaxis); hyperpigmentation or hypopigmentation of the skin; atrophy of the skin and subcutaneous tissue; exacerbation of the condition after injection into the synovial cavity; Charcot arthropathy; infection of the injection site due to non-compliance with the rules of asepsis and antisepsis; sterile abscess. Patients receiving GCS therapy may develop Kaposi's sarcoma. For clinical remission of this disease, the drug should be discontinued.