Betaspan Depot suspension for injection 7 mg/ml 1 ml ampoule 1 pc. in Moscow
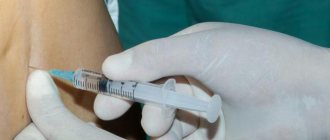
IM, intra-articular, periarticular, intrabursal, intramuscular, interstitial and intralesional administration.
The small size of betamethasone dipropionate crystals allows the use of small diameter needles (up to 26 gauge) for intravenous administration and injection directly into the lesion.
Cannot be administered intravenously or subcutaneously.
Strict adherence to aseptic rules is mandatory when using the drug.
The syringe should be shaken before administering the drug.
The dosage regimen and route of administration are determined individually, depending on the indications, severity of the disease and patient response.
For systemic therapy, the initial dose of Betaspan® Depot in most cases is 1–2 ml. The administration is repeated as necessary, depending on the patient's condition.
IM injection
IM administration of Betaspan® Depot should be carried out deep into the muscle, choosing large muscles and avoiding contact with other tissues (to prevent tissue atrophy).
In severe conditions requiring emergency measures, the initial dose is 2 ml of the drug.
For various dermatological diseases, as a rule, 1 ml of the drug is sufficient.
For diseases of the respiratory system, the onset of action of the drug occurs within several hours after intramuscular injection.
For bronchial asthma, hay fever, allergic bronchitis and allergic rhinitis, a significant improvement in the condition is achieved after administration of 1-2 ml of the drug.
For acute and chronic bursitis, the initial dose for intramuscular administration is 1–2 ml of the drug. If necessary, several repeated injections are performed.
If a satisfactory clinical response does not occur after a certain period of time, the drug should be discontinued and other therapy should be prescribed.
Local administration
When administered locally, simultaneous use of a local anesthetic drug is necessary only in rare cases. If it is desired, then use 1 or 2% solutions of procaine hydrochloride or lidocaine that do not contain methylparaben, propylparaben, phenol and other similar substances. In this case, mixing is carried out in a syringe, first drawing the required dose of Betaspan® Depot into the syringe from the bottle. Then the required amount of local anesthetic is taken from the ampoule into the same syringe and shaken for a short period of time.
For acute bursitis (subdeltoid, subscapular, ulnar and prepatellar), injection of 1–2 ml of the drug into the synovial bursa relieves pain and restores joint mobility within several hours. After stopping the exacerbation of chronic bursitis, smaller doses of the drug are used.
For acute tenosynovitis, tendinitis and peritendinitis, one injection of the drug improves the patient’s condition; for chronic cases, the injection is repeated depending on the patient’s response. Injecting the drug directly into the tendon should be avoided.
Intra-articular administration of the drug in a dose of 0.5-2 ml relieves pain and limited joint mobility in rheumatoid arthritis and osteoarthritis within 2-4 hours after administration. The duration of therapeutic action varies significantly and can be 4 or more weeks.
Recommended doses of the drug when administered into large joints range from 1 to 2 ml; medium - 0.5–1 ml; in small ones - 0.25–0.5 ml.
For some dermatological diseases, intravenous injection of the drug directly into the lesion is effective; the dose is 0.2 ml/cm2. The lesion is punctured evenly using a tuberculin syringe and a needle with a diameter of about 0.9 mm. The total amount of the drug administered in all areas should not exceed 1 ml for 1 week. For injection into the lesion, it is recommended to use a tuberculin syringe with a 26-gauge needle.
Recommended single doses of the drug (with an interval between injections of 1 week) for bursitis: for callus - 0.25–0.5 ml (as a rule, 2 injections are effective), for a spur - 0.5 ml, for limited mobility of the big toe - 0.5 ml, for a synovial cyst - 0.25-0.5 ml, for tenosynovitis - 0.5 ml, for acute gouty arthritis - 0.5-1 ml. For most injections, a tuberculin syringe with a 25-gauge needle is suitable.
After achieving a therapeutic effect, the maintenance dose is selected by gradually reducing the dose of Betaspan® Depot, administered at appropriate intervals. The reduction is continued until the minimum effective dose is reached.
If a stressful situation (not related to the disease) occurs or threatens to occur, it may be necessary to increase the dose of the drug. Discontinuation of the drug after long-term therapy is carried out by gradually reducing the dose. The patient's condition is monitored for at least a year after the end of long-term therapy or use in high doses.
Betaspan® Depo
Severe nervous system complications (including death) have been reported with epidural and intrathecal administration of GCS (with or without fluoroscopic guidance), including spinal cord infarction, paraplegia, quadriplegia, cortical blindness and stroke. Since the safety and effectiveness of epidural corticosteroids have not been established, this route of administration is not indicated for this group of drugs.
Recommended routes of administration are listed in the "Method of administration and dosage" section. Intravascular administration of the drug must be avoided. Due to the lack of data regarding the risk of calcification, injection of the drug into the intervertebral space is contraindicated.
The dosage regimen and route of administration are determined individually, depending on the indications, severity of the disease and patient response.
The dose should be as small as possible and the period of use as short as possible.
The initial dose is adjusted until the desired therapeutic effect is achieved. Then gradually reduce the dose of Betaspan® Depot to the minimum effective maintenance dose. If there is no effect from the therapy or if it is used for a long time, the drug is also discontinued, gradually reducing the dose.
Gradual withdrawal of GCS can reduce the risk of developing secondary adrenal insufficiency.
If a stressful situation (not related to the disease) occurs or threatens to occur, it may be necessary to increase the dose of Betaspan® Depot; the drugs of choice as a supplement should be hydrocortisone and cortisone.
The development of secondary adrenal insufficiency due to too rapid withdrawal of GCS is possible within several months after the end of therapy. If a stressful situation occurs or threatens to occur during this period, therapy with Betaspan® Depot should be resumed and a mineralocorticosteroid drug should be prescribed at the same time (due to a possible disturbance in the secretion of mineralocorticosteroids).
The patient's condition is monitored for at least one year after completion of long-term therapy or use in high doses.
Administration of the drug into soft tissues, into the lesion and intra-articularly can, with a pronounced local effect, simultaneously lead to a systemic effect.
Considering the likelihood of developing anaphylactoid reactions with parenteral administration of GCS, the necessary precautions should be taken before administering the drug, especially if the patient has a history of allergic reactions to drugs.
The drug Betaspan® Depot contains two active substances - betamethasone derivatives, one of which - betamethasone sodium phosphate - quickly penetrates into the systemic circulation, and therefore its possible systemic effect should be taken into account.
When using the drug, mental disorders are possible (especially in patients with emotional lability or a tendency to psychosis).
The effect of GCS is enhanced in patients with liver cirrhosis or hypothyroidism.
When using Betaspan® Depot in patients with diabetes mellitus, adjustment of hypoglycemic therapy may be required.
Patients receiving corticosteroids should not be vaccinated against smallpox. Other immunizations should not be carried out in patients receiving GCS (especially in high doses), due to the possibility of developing neurological complications and a low immune response (lack of antibody formation).
Betaspan® Depot should not be used 8 weeks before and 2 weeks after vaccination with killed or inactivated viral and antibacterial vaccines. However, immunization is possible during replacement therapy (for example, with primary adrenal insufficiency).
Patients receiving Betaspan® Depot in doses that suppress the immune system should be warned about the need to avoid contact with patients with chickenpox and measles (especially important when using the drug in children).
With the use of GCS, it is possible to suppress the reaction during skin tests.
When using the drug, it should be taken into account that GCS can mask the signs of an infectious disease, as well as reduce the body's resistance to infections.
The immunosuppressive effect of GCS can lead to activation of a latent infection or exacerbation of incurrent infections, including infections caused by microorganisms: Candida, Mycobacterium, Toxoplasma, Strongyloides, Pneumocystis, Cryptococcus, Nocardia
or
Ameba
.
Particular caution should be exercised when using GCS in patients with confirmed or suspected Strongyloides
(eel intestinalis).
In such patients, GCS-induced immunosuppression can lead to Strongyloides
and spread of infection through larval migration, which is often accompanied by severe enterocolitis and Gram-negative septicemia, possibly fatal.
Since corticosteroids may aggravate the course of latent amebiasis, all patients with unexplained diarrhea or patients arriving from countries with a tropical climate should be examined to exclude amebiasis before initiating corticosteroid therapy. It is necessary to carefully observe the rules of asepsis and antisepsis when administering the drug.
Caution must be exercised when using the drug in patients at high risk of infection (on hemodialysis or with dentures). The use of the drug for active tuberculosis is possible only in cases of fulminant or disseminated tuberculosis in combination with adequate anti-tuberculosis therapy. When using the drug in patients with latent tuberculosis or during the period of tuberculin testing, select the dose of the drug
Betaspan® Depot must be very careful (due to the danger of reactivation of tuberculosis), and with long-term use, anti-tuberculosis chemoprophylaxis is necessary.
When using rifampicin prophylactically, acceleration of the hepatic clearance of betamethasone should be taken into account (betamethasone dose adjustment may be required).
If there is fluid in the joint cavity, a septic process should be excluded. A noticeable increase in pain, swelling, increased temperature of the surrounding tissues and further limitation of joint mobility indicate septic arthritis. It is necessary to conduct a study of aspirated joint fluid. Once the diagnosis is confirmed, appropriate antibacterial therapy must be prescribed. The use of Betaspan® Depot for septic arthritis is contraindicated.
Repeated injections into a joint for osteoarthritis may increase the risk of joint destruction. The introduction of GCS into the tendon tissue gradually leads to tendon rupture. After successful intra-articular therapy, the patient should avoid overloading the joints.
Long-term use of GCS can lead to posterior subcapsular cataracts (especially in children), glaucoma with possible damage to the optic nerve and may contribute to the development of secondary eye infections (fungal or viral). Periodic ophthalmological examinations should be carried out, especially in patients receiving Betaspan® Depot for more than 6 weeks
Particular care must be taken when considering the possibility of systemic use of GCS in patients with active herpetic eye lesions (keratitis caused by the herpes simplex virus).
The use of medium and high doses of corticosteroids can lead to increased blood pressure, sodium and fluid retention in the body, and increased excretion of potassium from the body (these phenomena are less likely when taking synthetic corticosteroids, unless they are used in high doses). With long-term use of high doses of Betaspan® Depot, the risk of developing arrhythmia and hypokalemia should consider the need to prescribe potassium-containing drugs and a diet with limited salt. All corticosteroids enhance calcium excretion.
When using Betaspan® Depot simultaneously with cardiac glycosides or drugs that affect the electrolyte composition of plasma, monitoring of the water-electrolyte balance is required.
Use acetylsalicylic acid with caution in combination with Betaspan® Depot for hypoprothrombinemia.
Caution must be exercised when using GCS in elderly patients; in patients with renal or hepatic failure, diverticulitis, active or latent gastric and/or intestinal ulcers or the presence of recently created intestinal anastomoses, osteoporosis, confirmed or suspected parasitic infections (for example, strongyloidiasis).
Symptoms of peritoneal irritation or reduction in pain due to perforation of the walls of the stomach or intestines may be minimal or absent in patients receiving GCS.
GCS should be used with caution in patients with hypothyroidism or myasthenia gravis
.
Cases of Kaposi's sarcoma have been reported in patients receiving corticosteroids; discontinuation of this therapy can lead to remission of the disease.
With the use of corticosteroids, changes in sperm motility and number are possible.
During long-term therapy with GCS, it is advisable to consider the possibility of switching from parenteral to oral use of GCS, taking into account the assessment of the benefit/risk ratio.
Use in pediatrics
Children undergoing drug therapy (especially long-term therapy) should be under close medical supervision for possible growth retardation and the development of secondary adrenal insufficiency.
Use in athletes
Patients participating in competitions under the control of the World Anti-Doping Agency (WADA) should familiarize themselves with the WADA rules before starting treatment with the drug, since taking Betaspan® Depot may affect the results of doping control.
Description of the drug BETASPAN DEPO® (BETASPAN DEPO®)
GKS. It has anti-inflammatory, antiallergic, desensitizing, antishock, antitoxic and immunosuppressive effects, and also has a pronounced and diverse effect on various types of metabolism.
Suppresses the release of ACTH and beta-lipotropin by the pituitary gland, but does not reduce the level of circulating beta-endorphin. Inhibits the secretion of TSH and FSH.
Increases the excitability of the central nervous system, reduces the number of lymphocytes and eosinophils, increases the number of red blood cells (stimulates the production of erythropoietin).
Interacts with specific cytoplasmic receptors and forms a complex that penetrates the cell nucleus and stimulates the synthesis of mRNA; the latter induces the formation of proteins, incl. lipocortin, mediating cellular effects. Lipocortin inhibits phospholipase A2, suppresses the release of arachidonic acid and suppresses the synthesis of endoperoxides, prostaglandins, leukotrienes, which contribute to inflammation and allergies.
The anti-inflammatory effect is associated with inhibition of the release of inflammatory mediators by eosinophils; inducing the formation of lipocortin and reducing the number of mast cells that produce hyaluronic acid; with a decrease in capillary permeability; stabilization of cell membranes and organelle membranes (especially lysosomal ones).
The antiallergic effect develops as a result of suppression of the synthesis and secretion of allergy mediators, inhibition of the release of histamine and other biologically active substances from sensitized mast cells and basophils, T- and B-lymphocytes, mast cells, decreased sensitivity of effector cells to allergy mediators, inhibition of antibody formation, changes in immune body response.
Antishock and antitoxic effects are associated with an increase in blood pressure (due to an increase in the concentration of circulating catecholamines and restoration of the sensitivity of adrenergic receptors to them, as well as vasoconstriction), a decrease in the permeability of the vascular wall, membrane protective properties, and activation of liver enzymes involved in the metabolism of endo- and xenobiotics.
In COPD, the action is based mainly on inhibition of inflammatory processes, inhibition of development or prevention of swelling of the mucous membranes, inhibition of eosinophilic infiltration of the submucosal layer of the bronchial epithelium, deposition of circulating immune complexes in the bronchial mucosa, as well as inhibition of erosion and desquamation of the mucous membrane. Increases the sensitivity of beta-adrenergic receptors of small and medium-caliber bronchi to endogenous catecholamines and exogenous sympathomimetics, reduces the viscosity of mucus by inhibiting or reducing its production.
The immunosuppressive effect is due to inhibition of the release of cytokines (interleukin-1, interleukin-2, interferon gamma) from lymphocytes and macrophages.
Suppresses the synthesis and secretion of ACTH and, secondarily, the synthesis of endogenous corticosteroids. Inhibits connective tissue reactions during the inflammatory process and reduces the possibility of scar tissue formation.
Protein metabolism:
- reduces the amount of protein in plasma (due to globulins) with an increase in the albumin/globulin ratio, increases the synthesis of albumins in the liver and kidneys;
- enhances protein catabolism in muscle tissue.
Lipid metabolism:
- increases the synthesis of higher fatty acids and TG, redistributes fat (fat accumulation mainly in the shoulder girdle, face, abdomen), leads to the development of hypercholesterolemia.
Carbohydrate metabolism:
- increases the absorption of carbohydrates from the gastrointestinal tract;
- increases the activity of glucose-6-phosphatase, leading to an increase in the flow of glucose from the liver into the blood;
- increases the activity of phosphoenolpyruvate carboxylase and the synthesis of aminotransferases, leading to the activation of gluconeogenesis.
Water-electrolyte metabolism:
- retains sodium and water in the body, stimulates the excretion of potassium, reduces the absorption of calcium from the gastrointestinal tract, “washes” calcium from the bones, and increases excretion by the kidneys.
Betamethasone sodium phosphate
is an easily soluble compound that is well absorbed after parenteral administration into tissues and provides a rapid effect.
Betamethasone dipropionate
has slower absorption. By combining these salts it is possible to create medicines with both short-term (but fast) and long-term effects. Depending on the method of application (IV, IM, intra-articular, periarticular, IV), a general or local effect is achieved.