Indications:
- progressive prostate cancer (palliative treatment), incl. when orchiectomy or estrogen treatment is not indicated or appropriate for the patient;
- endometriosis (for a period of up to 6 months as primary therapy or in addition to surgical treatment);
- uterine fibroids (for a period of up to 6 months as preoperative preparation for removal of fibroids or hysterectomy, as well as for symptomatic treatment and improvement of condition in women during menopause who refuse surgical intervention);
- breast cancer in the perimenopausal period in combination with hormone therapy;
- children with precocious puberty (PPS) of central origin.
O.G. Sukonko, S.L. Polyakov, S.A. Krasny, A.G. Zhegalik, O.A. Agafonov, A.I. Rolevich
State Institution “Research Institute of Oncology and Medical Radiology named after. N.N. Alexandrova”
Key words: prostate cancer, luteinizing hormone-releasing hormone analogues, luprid depot
SUMMARY
PURPOSE: To evaluate the clinical efficacy and toxicity of lupride depot (leuprorelin acetate, Sun Pharm. Ind. Ltd.).
MATERIAL AND METHODS: The results of treatment of 30 patients with prostate cancer treated at the State Research Institute of OMR named after. N.N. Alexandrov from March 2005 to June 2007 and receiving hormonal therapy with lupride depot. The decrease in PSA level compared to the baseline, the dynamics of symptoms associated with the presence of a tumor, as well as side effects of therapy were analyzed.
RESULTS: Treatment with lupride depot resulted in a 74% to 99% reduction in PSA levels from baseline, depending on tumor extent. Pain associated with the presence of bone metastases decreased or was completely relieved in all 5 patients with symptomatic bone metastases. 6 out of 7 patients with lower urinary tract symptoms achieved significant improvement in IPSS scores. Side effects of hormone therapy with lupride depot included the usual symptoms of androgen deficiency.
CONCLUSIONS: Lupride depot is effective in patients with hormone-sensitive prostate cancer in terms of reducing PSA levels, as well as relieving tumor-related symptoms.
INTRODUCTION
The incidence of prostate cancer (PCa) in the Republic of Belarus is growing rapidly. Currently, prostate cancer ranks first in terms of growth rate among all malignant neoplasms. Over the past ten years in the Republic of Belarus, the number of annually registered cases of prostate cancer has increased from 932 in 1996 to 1681 cases in 2005 (1.8 times) [1]. The incidence since 1996 has increased from 19.3 to 36.7 per 100,000 population in 2005. Currently, prostate cancer occupies 9.2% of the structure of cancer incidence in the Republic of Belarus and ranks 4th after lung, skin and stomach cancer [2 ].
One of the effective methods of treating prostate cancer remains hormonal therapy, which consists in eliminating the effect of androgens in the male body. This method of treatment, previously used mainly for metastatic prostate cancer, has proven its effectiveness in locally advanced forms of the tumor [3, 4]. One of the effective, low-toxicity and non-traumatic methods of hormonal therapy for prostate cancer is medical castration using analogs of luteinizing hormone releasing hormone (LHRH). A new representative of this class of drugs is “Lupride Depot” (leuprorelin acetate, Sun Pharmaceutical Industries Ltd.), which recently appeared in the Republic of Belarus. This study was conducted to evaluate the clinical effectiveness and toxicity of lupride depot based on the experience of using the drug at the State Research Institute of OMR named after. N.N. Alexandrova.
MATERIAL AND METHODS
The results of treatment of 30 patients with prostate cancer treated at the State Research Institute of OMR named after. N.N. Alexandrov from March 2005 to June 2007 and receiving hormonal therapy with lupride depot. The characteristics of the patients are presented in Table 1.
The effectiveness of treatment, defined as a decrease in the level of prostate-specific antigen (PSA) in relation to the initial level or the dynamics of symptoms associated with the presence of a tumor, as well as side effects of therapy were analyzed.
RESULTS
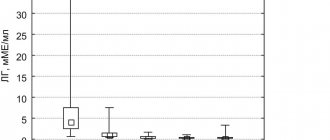
The effectiveness of hormone therapy with lupride depot in reducing PSA levels is shown in Table 2. In general, treatment with lupride depot led to a decrease in PSA levels by 74-99% from baseline.
The dynamics of symptoms associated with prostate cancer were assessed in patients with clinical manifestations of prostate cancer at the time of initiation of therapy. Pain associated with the presence of bone metastases decreased or was completely relieved in all 5 patients with symptomatic bone metastases. 6 out of 7 (85.7%) patients with lower urinary tract symptoms achieved significant improvement in IPSS (International Prostatic Symptom Score) after a 3-6 month course of lupride depot.
Side effects of hormone therapy with lupride depot included symptoms of androgen deficiency, usually developing against the background of an effective decrease in testosterone levels in the blood: decreased libido and potency, general weakness, fatigue, anemia, loss of muscle strength, osteoporosis, hot flashes and gynecomastia.
DISCUSSION AND CONCLUSIONS
Hormonal treatment of prostate cancer became widely used after the work of S. Huggins and S. Hodges (1941), who showed that hormonal treatment in the form of castration or estrogen administration leads to relief of prostate cancer symptoms and normalization of alkaline phosphatase levels [5].
The mechanisms of regulation of sex hormone production in men have been studied in detail [6] and are briefly described below. The largest amount of androgens, mainly testosterone, is produced in the testicles by Leydig cells, the remaining 5-10% of androgens are produced in the adrenal glands. The functioning of Leydig cells is regulated by luteinizing hormone (LH) from the pituitary gland, the production of which in turn is stimulated by LHRH produced in the hypothalamus. There is a negative feedback mechanism where low levels of testosterone in the blood stimulate the production of LHRH, which leads to increased production of LH and increased testosterone levels. The secretion of androgens by the adrenal glands is regulated by adrenocorticotropic hormone. The synthesis of the most active androgen, 5α-dihydrotestosterone, occurs through the transformation of testosterone with the participation of the enzyme 5α-reductase contained in prostate tissue. Androgens exert their action by binding to an intracellular receptor, after which the receptor-hormone complex interacts with certain sections of DNA, stimulating its transcription.
Currently, there are a number of methods available to influence this system in order to deprive PCa cells of androgen stimulation, which include surgical or drug castration, androgen blockade at the level of target cells [7], maximum [8] and intermittent androgen blockade [9].
Bilateral orchiectomy quickly reduces the level of androgens in the blood to very small values (the so-called castration level of testosterone) by eliminating Leydig cells from the patient’s body. This is a fairly low-traumatic, effective and cheap method of hormonal treatment, however, a large number of patients, due to mainly psychological reasons, prefer medical methods of castration [10].
In 1971, A. Schally [11] described the structure of the LHRH molecule, for which he was awarded the Nobel Prize. This prompted the development of a number of peptides with properties that stimulate or block LHRH receptors. Currently, the most widely used analogues of LHRH are those that have a greater affinity for the receptor than endogenous LHRH. Binding of the LHRH analogue to the receptor in the pituitary gland leads to the release of LH with subsequent depletion of its production, which leads to an initial rise and then a decrease in blood testosterone levels to castration levels. This initial stimulation of LH and testosterone production is called the “flare” phenomenon. Clinically, in a patient with advanced prostate cancer, this phenomenon can manifest itself as an exacerbation of symptoms during the first few weeks of treatment, increased bone pain and can sometimes lead to pathological bone fractures and even death [12]. Therefore, to prevent such complications, it is recommended to prescribe an antiandrogen simultaneously or 7 days before the administration of an LHRH analogue and continue taking it during the first 2 to 4 weeks of treatment [13]. The most commonly used LHRH analogues are goserelin (Zoladex), luprorelin (Lupron, Viadur, Eligard), triptorelin (Diferelin, Trelstar Depot), and buserelin (Superfact). Depot forms of these drugs have been created, requiring administration once every 4 or 13 weeks [14]. The effectiveness of LHRH analogues is approximately equal to the effectiveness of surgical castration, the same can be said about side effects [15-16]. This allowed us to consider this method of hormone therapy “standard” in the treatment of advanced prostate cancer. In addition, long-term therapy with LHRH analogs is the only hormone therapy indicated for patients with locally advanced prostate cancer in combination with external beam radiation therapy. The advantage of using LHRH analogues is the psychological effect of preserving the testicles, as well as the complete reversibility of the effect upon discontinuation of the drug. The disadvantage of their use is the high cost of treatment, but the use of cheaper generic drugs can reduce costs. Table 3 shows the comparative cost of various LHRH analogues registered in the Republic of Belarus.
The present study did not examine the level of testosterone in the blood of patients with prostate cancer treated with lupride depot, although obtaining this data seems very relevant. Previously, a castration testosterone level was considered to be 50 ng/dL (1.7 nmol/L) [17]. More recent studies examining testosterone levels after surgical castration using chemiluminescence, which provides a more accurate measurement of testosterone levels, found levels to be around 20 ng/dL (0.7 nmol/L) [17–18]. This led to a decrease in testosterone levels, which is considered castration. Currently, most experts believe that LHRH analogues should provide and maintain this level [19].
Thus, studying the effect of lupride depot on clinical parameters demonstrated its effectiveness in reducing PSA levels and also relieving tumor-related symptoms in patients with hormone-sensitive prostate cancer. Further studies are needed to confirm the effective reduction of testosterone levels in patients treated with lupride depot.
LITERATURE
- Epidemiology of malignant neoplasms in Belarus / I.V. Zalutsky [and others]. - Minsk: Zorny verasen, 2006. - 207 p.
- Polyakov, S.M. Malignant neoplasms in Belarus, 1996-2005 / S.M. Polyakov, L.F. Levin, N.G. Shebeko; Ed. A.A. Grakovich, I.V. Zalutsky. - Minsk: BELTsMT, 2006. - 194 p.
- Bolla, M., Gonzalez, D., Warde P. et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. // N. Engl. J. Med—1997.—Vol. 337.— P. 295—300.
- Pilepich, M. V., Caplan, R. W., Byhardt, R. W. et al. Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: Report of Radiation Therapy Oncology Group Protocol 85-31. // J. Clin. Oncol.—1997.—Vol. 15.— P. 1013—1021.
- Huggins, C., Hodges, C. V. Studies on prostatic cancer. I. The effect of castration, estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. // Cancer Res. - 1941. - Vol. 1.— P. 293—297.
- Schroder, F.H. Endocrine treatment of prostate cancer. In: Walsh, P. C., Retik, A. B., Vaughan, E. D. Jr., Wein, A. J. (eds.). Campbell's Urology.—7th ed.—Baltimore: WB Saunders, 1998.—P. 2636–2638.
- McLeod, D.G., Iversen, P., See, W.A. and Casodex Early Prostate Cancer Trialists' Group. Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. // BJU Int.—2006.—Vol. 97.— P. 247—254.
- Prostate Cancer Trialists' Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomized trials. // Lancet—2000.—Vol. 355.— P. 1491—1498.
- Kienle, E.F., Hillger, H. Can intermittent androgen deprivation be an alternative to continuous androgen withdrawal in patients with psa-relapse? First results of the randomized prospective phase-III clinical trial EC 507. // J. Urol.— 2003.— Vol. 169.—P. 396 (Abstract 1481).
- Cassileth, BR, Soloway, MS, Vogelzang, NJ et al. Patients' choice of treatment in stage D prostate cancer. // Urology— 1989.— Vol. 33.— P. 57—62.
- Schally, A.V., Arimura, A., Baba, Y. et al. Isolation and properties of the FSH and LH-releasing hormone. // Biochem. Biophys. Res. Commun.—1971.—Vol. 43.— P. 393—399.
- Thompson, IM, Zeidman, EJ, Rodriguez, FR Sudden death due to disease flare with luteinizing hormone-releasing agonist therapy for carcinoma of the prostate. // J. Urol.— 1990.— Vol. 144.— P. 1479—1480.
- Boccon-Gibod, L. The prevention of LHRH induced disease flares in patients with metastatic carcinoma of the prostate. // Prog. Clin. Biol. Res.—1990.—Vol. 359.— P. 125—129.
- Sarosdy, M.F., Schellhammer, P.F., Soloway, M.S. et al. Endocrine effects, efficacy and tolerability of a 10.8-mg depot formulation of goserelin acetate administered every 13 weeks to patients with advanced prostate cancer. // BJU Int. - 1999. - Vol. 83.— P. 801—806.
- Soloway, MS, Chodak, G., Vogelzang, NJ et al. Zoladex versus orchiectomy in treatment of advanced prostate cancer: A randomized trial. Zoladex Prostate Study Group. // Urology— 1991.— Vol. 37.— P. 46—51.
- de Voogt, H. J., Studer, U., Schroder, F. H. et al. Maximum androgen blockade using LHRH agonist buserelin in combination with short-term (two weeks) or long-term (continuous) cyproterone acetate is not superior to standard androgen deprivation in the treatment of advanced prostate cancer. Final analysis. //Eur. Urol.— 1998.— Vol. 33.— P. 152—158.
- Oefelein, M. G., Feng, A., Scolieri, M. J. et al. Reassessment of the definition of castrate levels of testosterone: implications for clinical decision making. // Urology—2000.—Vol. 56.— P. 1021—1024.
- Rohl, HF, Beuke, HP Effect of orchidectomy on serum concentrations of testosterone and dihydrotestosterone in patients with prostatic cancer. // Scand. J. Urol. Nephrol.— 1992.— Vol. 26.— P. 11—14.
- Zlotta, A., Debruyne, F. Expert consultation on optimal testosterone control in prostate cancer. //Eur. Urol. Suppl.—2005.—Vol. 4.— P. 37—41.
Table 1 - Characteristics
| Index | Meaning |
| Average age (M±m) | 69,5±9,6 |
| Process prevalence (n, %): | |
| Localized PCa (T1-2N0M0) | 7 (23,3%) |
| Locally advanced prostate cancer: T3N0M0 T2-4N1M0 | 6 (20,0%) 3 (10,0%) |
| Metastatic PCa (T2-4N0-1M1) | 14 (46,7%) |
| PSA level before treatment, ng/ml (M, max–min) | 273,5 (13–2849) |
| Degree of differentiation (according to Gleason) (n, %): | |
| 2-4 | 6 (20,0) |
| 5-6 | 9 (30,0) |
| 7-10 | 15 (50,0) |
| Note: n – number of patients; M – arithmetic mean; m – standard deviation | |
Table 2 - PSA dynamics depending on the degree of tumor spread
| Index | n | Initial PSA level, ng/ml (M±m) | PSA level after treatment, ng/ml (M±m) | Dynamics of PSA level, % to initial level |
| Localized PCa (T1-2N0M0) | 7 | 11,1±6,4 | 3,0±2,8 | — 74,0 |
| Locally advanced prostate cancer (T2-4N0-1M0) | 9 | 44,6±41,1 | 3,4±3,7 | — 92,3 |
| Metastatic PCa (T2-4N0-1M1) | 14 | 678,4±432,8 | 1,2±0,9 | — 99,9 |
Note: n – number of patients; M – arithmetic mean; m – standard deviation
Table 3 - Comparative characteristics of various LHRH analogues
| A drug | Manufacturer | Active substance | Dose | Cost, rub.* |
| Zoladex | Astra Zeneca | Goserelin acetate | 3.6 mg | 526 000 |
| Diferelin | Beaufour Ipsen Int. | Triptorelin | 3.75 mg | 431 000 |
| Luprid depot | Sun Pharm. Ind. | Leuprorelin acetate | 3.75 mg | 251 000 |
Note: * wholesale price for pharmacies according to the Belarusian expert organization “Pharmexpert”
Contraindications:
- hypersensitivity to leuprorelin, similar drugs of protein origin or to any other excipient included in the dosage form;
- surgical castration;
- pregnancy;
- breastfeeding period;
- vaginal bleeding of unknown etiology;
- prostate cancer (hormone independent);
- children's age (up to 18 years), except for children with PPS of central origin.
- women over 65 years of age.
With caution: patients with spinal metastases, urinary tract obstruction or hematuria.
Instructions for use of LUPRIDE DEPOT
Adverse reactions are presented by organs and organ systems and by frequency of occurrence:
- very often - more than 10%, often - 1-10%, infrequently - 0.1-1%, single - 0.01-0.1%, rare - less than 0.01%.
Adverse reactions reported in clinical studies
Prostate cancer
Most patients experienced an increase in testosterone levels during the first week, decreasing to baseline levels or lower until the end of the second week of treatment. During the first few weeks of treatment, symptoms may worsen in patients with spinal metastases and/or urinary tract obstruction or hematuria, which, if progressing, may lead to neurological symptoms such as temporary weakness and/or paresthesia of the lower extremities or worsening of symptoms with sides of the urinary tract.
The following adverse reactions were noted during clinical studies and had a possible or probable relationship with the drug.
| Organ system | Adverse reactions with a frequency of 5% or more | Adverse reactions with a frequency of less than 5% |
| Cardiac disorders | Edema | Angina pectoris, arrhythmia |
| Gastrointestinal disorders | Nausea, vomiting | Anorexia, diarrhea |
| Endocrine disorders | Reduced testicular size, hot flashes/sweating, impotence (physiological effects of decreased testosterone levels) | Gynecomastia, decreased libido |
| Neurological and mental disorders | General pain | Paresthesia, insomnia |
| Respiratory system disorder | Dyspnea | Hemoptysis |
| Musculoskeletal and connective tissue disorders | Ossalgia, myalgia | |
| Disorders of the genitourinary system | Dysuria, hematuria, testicular pain | |
| Changes in the skin and subcutaneous tissue | Dermatitis, local skin reactions, hypertrichosis | |
| Other | Asthenia | Diabetes, flu-like syndrome, weight gain, lump in throat, hypercalcemia, hyperuricemia |
Endometriosis
Estradiol levels may increase during the first weeks after the first injection, but then decrease to menopausal levels in patients with endometriosis/uterine fibroids. A transient increase in estradiol levels may be associated with a temporary exacerbation of symptoms.
The following adverse reactions were recorded during clinical trials and had a possible or probable relationship with the drug.
| Organ system | Adverse reactions with a frequency of 5% or more | Adverse reactions with a frequency of less than 5% |
| Cardiac disorders | swelling | Increased heart rate, syncope, tachycardia. |
| Gastrointestinal disorders | Nausea, vomiting | Dry mouth, thirst, change in appetite |
| Endocrine disorders | Hot flushes/sweating, breast pain, decreased libido (physiological effects of decreased estrogen levels), androgen-like effects, acne, seborrhea, hirsutism, voice changes | — |
| Neurological and mental disorders | Depression/emotional lability, headache, neuromuscular disorders, sleep disturbance/insomnia, nervousness (physiological effects of decreased estrogen levels), dizziness, general pain, paresthesia | Personality changes, memory impairment, hallucinations. |
| Musculoskeletal and connective tissue disorders | Myalgia, arthralgia (physiological effects of decreased estrogen levels) | — |
| Disorders of the genitourinary system | Vaginitis (physiological effect of decreased estrogen levels) | Dysuria, lactation. |
| Changes in the skin and subcutaneous tissue | Skin reactions | Ecchymosis, alopecia, hair growth disorder. |
| Other | Asthenia, increase/decrease in body weight | Ophthalmic disorders, lymphadenopathy |
Uterine fibroids
The following adverse reactions were recorded during clinical trials and had a possible or probable relationship with the drug
| Organ system | Adverse reactions with a frequency of 5% or more | Adverse reactions with a frequency of less than 5% |
| Cardiac disorders | Edema | — |
| Gastrointestinal disorders | Nausea, vomiting | Dry mouth, diarrhea, constipation, thirst, increased appetite, flatulence |
| Endocrine disorders | Hot flashes/sweating (physiological effects of decreased estrogen levels) | Decreased libido, breast pain |
| Neurological and mental disorders | Depression/emotional lability, headache (physiological effects of decreased estrogen levels), insomnia, general pain, dizziness | Nervousness, paresthesia. |
| Musculoskeletal and connective tissue disorders | Arthralgia | Arthralgia, increased muscle tone |
| Disorders of the genitourinary system | Vaginitis (physiological effect of decreased estrogen levels) | |
| Changes in the skin and subcutaneous tissue | Local skin reactions | Changing nails |
| Other | Asthenia | Increase/decrease in body weight, taste sensitivity disorders, flu-like syndrome, vaginal odor |
Adverse reactions observed in children in clinical studies
| Organ system | Adverse reactions with a frequency of 2% or more | Adverse reactions with a frequency of less than 2% |
| Cardiac disorders | Vasodilation | Bradycardia, hypertension, peripheral vascular disorders, syncope |
| Gastrointestinal disorders | Nausea/vomiting, dysphagia, gingivitis, constipation, dyspepsia, increased appetite | |
| Respiratory system disorders | — | Asthma, nosebleeds, rhinitis, sinusitis |
| Endocrine disorders | — | Accelerated puberty, feminization, goiter |
| Metabolism disorder | Peripheral edema, weight gain, growth retardation | |
| Neurological and mental disorders | General pain, emotional lability, headache | Irritability, drowsiness, depression, hyperkinesia |
| Musculoskeletal and connective tissue disorders | Arthralgia, myalgia, myopathy, joint dysfunction | |
| Disorders of the genitourinary system | Vaginitis, vaginal bleeding, leucorrhoea | Gynecomastia, urinary incontinence, cervical disorders/neoplasms, dysmenorrhea, menstrual/breast disorders |
| Changes in the skin and subcutaneous tissue | Acne, seborrhea, injection site reaction including abscess (most reactions were mild or moderate), rash including erythema multiforme. | Alopecia, hair growth disorder, nail growth disorder, hirsutism, leucoderma, skin hypertrophy, purpura |
| General disorders, changes in laboratory parameters | Aggravation of an existing tumor, decreased vision, allergic reaction, fever, flu-like symptoms, infectious diseases, the appearance of antinuclear antibodies, increased ESR. |
Post-marketing data (all age groups)
Given the impossibility of accurately counting the number of patients during post-marketing use, data on adverse reactions are provided without calculating their frequency. Since leuprolide has several indications for use in different patient populations, some adverse reactions may not affect every individual patient.
For most adverse reactions, a causal relationship with the drug has not been established.
Cardiac disorders
Angina pectoris, bradycardia, arrhythmia, chronic heart failure, changes in the electrocardiogram (ECG), increase/decrease in blood pressure, auscultatory noises, myocardial infarction, phlebitis, pulmonary embolism, stroke, syncope, tachycardia, thrombosis, transient ischemic attacks, varicose veins .
Gastrointestinal disorders
Diarrhea or constipation, dry mouth, duodenal ulcer, gastrointestinal bleeding, liver dysfunction, increased appetite, changes in liver enzymes, nausea, stomach ulcers, rectal polyps, thirst, vomiting.
Endocrine disorders
Diabetes, enlarged thyroid gland, and, as with the use of other drugs in this group, very rare cases of hemorrhage into the pituitary gland were observed after the first prescription in patients with pituitary adenoma.
Blood and lymphatic system disorders
Anemia, ecchymosis, increased prothrombin and thromboplastin times, thrombocytopenia, leukocytosis, leukopenia.
Metabolism disorder
Increased blood urea levels, hypercalcemia and hypercreatinemia, dehydration, edema, hyperlipidemia (increased total cholesterol, low-density lipoproteins, triglycerides), hyperphosphatemia, hypoglycemia, hypoproteinemia, hyponatremia, hyperuricemia, hyperbilirubinemia, isolated cases of anaphylaxis.
Musculoskeletal and connective tissue disorders
Ankylosing spondylitis, arthralgia, myalgia, pelvic fibrosis, vertebral fracture, tenosynovitis-like symptoms, ossalgia.
Neurological and mental disorders
Hallucinations, dizziness, lightheadedness, hypoesthesia, insomnia, lethargy, increased libido, memory impairment, mood changes, nervousness, neuromuscular disorders, numbness, paresthesia, peripheral neuropathy, sleep disturbance, taste disturbances, convulsions. Behavioral changes and depression were observed with long-term use of leuprorelin in 1-10% of cases, and with short-term use in 0.1-1% of cases. Very rarely, there were cases of patients having thoughts of suicide and suicide attempts. If these phenomena occur, the patient should immediately inform the attending physician.
Respiratory system disorders
Cough, shortness of breath, nosebleeds, pharyngitis, pleural effusion, pleural friction noise, pneumonia, fibrous formations in the lungs, infiltrates in the lungs, respiratory disorders, congestion in the paranasal sinuses.
Changes in the skin and subcutaneous tissue
Skin/ear carcinoma, dermatitis, dry skin, itching, increased/decreased hair growth, lump in throat, pigmentation, skin damage, urticaria. Reactions at the injection site (inflammation, sterile abscess, tissue compaction, hematoma).
Visual disorders
Visual impairment, amblyopia, blurred vision, ophthalmological disorders, dry eye.
Disorders of the hearing and vestibular apparatus
Hearing impairment, tinnitus.
Disorders of the genitourinary system
Bladder cramps, breast pain, breast tenderness, gynecomastia, hematuria, urinary incontinence, dysmenorrhea, including interruptions and continuous vaginal bleeding, swelling/penile dysfunction, prostate pain, testicular atrophy, testicular pain, decreased testicular size , urinary disorders (increased frequency, obstruction, urgency), genitourinary tract infections.
General disorders
Bloating, asthenia, fever, chills, general pain, headache, infectious diseases, symptoms of inflammation, photosensitivity reactions, swelling in the temporal bone, jaundice.
Directions for use and dosage:
IM or s/c, 1 time per month. The injection site should be changed periodically. The injection suspension is prepared immediately before administration using the supplied solvent at a concentration of 3.75 mg/1 ml.
- For prostate or breast cancer, a single dose is 3.75 mg. The duration of treatment is determined by the doctor.
- For endometriosis and uterine fibroids, a single dose is 3.75 mg.
- For women of reproductive age, the first injection is given on the 3rd day of menstruation. The duration of treatment is no more than 6 months.
- For PPS in children, the initial dose is 0.3 mg/kg (minimum 7.5 mg) once every 4 weeks.
- Maintenance dose for PPS: If complete suppression of disease progression is not achieved, the dose should be increased every 4 weeks by 3.75 mg.
Discontinuation of Lucrin Depot should be considered before the age of 11 years in girls and 12 years of age in boys.
Lucrine depot
Trade name: Lucrin depot International name: Leuprorelin
Release form: lyophilisate for the preparation of suspension for injection 3.75 mg (bottles) / complete with solvent (ampoules) 2 ml, napkin, syringe and injection needles/
Composition: leuprorelin acetate 3.75 mg
Pharmacological group: antitumor agent - gonadotropin-releasing hormone analogue
Pharmacological group according to ATK: L02AE02 (Leuprorelin)
Pharmacological action: antitumor, gonadotropin-reducing production, pharmacologically castrating, cytostatic, gonadotropin-releasing hormone analogue, luteinizing hormone secretion-reducing, follicle-stimulating hormone secretion-reducing, androgen secretion-reducing, estrogen secretion-reducing, endometriosis treatment,
Indications: Progressive hormone-dependent prostate cancer (symptomatic treatment, as an alternative to orchiectomy or estrogen therapy). Endometriosis (laparoscopically confirmed). Uterine fibroids (during the period of preoperative preparation and as an alternative to surgical treatment of drugs).
Dosage regimen: IM or SC, once a month - the usual form and once every 3 months - the depot form (each time the injection site is changed - the skin of the abdomen, buttocks, thigh). For prostate cancer, a single dose is 3.75-7.5 mg. For uterine fibroids and endometriosis, a single dose is 3.75 mg. For women of reproductive age, the first injection is given on the 3rd day of menstruation. Duration of treatment - no more than 6 months. The depot form is administered intramuscularly or subcutaneously, once every 3 months (the injection site is changed - the skin of the abdomen, buttocks, thigh) at a dose of 11.25 mg. Solutions for injections are prepared immediately before administration.
Contraindications: Hypersensitivity, surgical castration, prostate cancer (hormone-independent), pregnancy, lactation.
Side effects: From the nervous system: headache, dizziness, fainting, sleep disturbances (including insomnia), nervousness, depression, anxiety, increased fatigue, paresthesia, hallucinations, stupor, memory impairment, personality changes, spinal cord compression (in men). From the cardiovascular system: palpitations, tachycardia, a feeling of compression in the chest, changes in the ECG. From the digestive system: changes in appetite, taste, dry mouth or hypersalivation, thirst, nausea, vomiting, weight gain or loss, diarrhea or constipation, increased activity of liver transaminases and alkaline phosphatase. From the senses: conjunctivitis, visual and hearing impairment, tinnitus. From the musculoskeletal system: in men - ossalgia, myasthenia in the legs, in women - osteoporosis (the process is reversible after completion of the course of treatment). From the genitourinary system: decreased testicular size, decreased potency, gynecomastia, decreased or increased size of the mammary glands, metrorrhagia (in the first week of treatment), decreased libido, dry vaginal mucosa, vaginitis, leucorrhoea. From the skin: dermatitis, itching, rash, ecchymosis, alopecia, in women - acne, hypertrichosis. Local reactions: compaction, hyperemia and pain at the injection site. Laboratory indicators: hypercholesterolemia (in women). Other: peripheral edema, changes in body odor, flu-like syndrome, “flushes” of blood to the skin of the face and upper chest, increased sweating, enlarged lymph nodes (in the first week of treatment), acute urinary retention (in men).
Pharmacodynamics: An antitumor agent, a synthetic analogue of endogenous GRF, has greater activity than the natural hormone. Interacts with pituitary gonadorelin receptors, causing their short-term stimulation followed by long-term inhibition of activity. Reversibly suppresses the release of LH and FSH by the pituitary gland, reduces the concentration of testosterone in the blood in men and estradiol in women, and causes receptor desensitization after short-term initial stimulation. After the first intramuscular injection for 1 week, the concentration of sex hormones in both men and women increases (physiological reaction). During the same period, the concentration of acid phosphatase in plasma increases, which is restored by 3-4 weeks of treatment. By 21 days after the first administration, the concentration of sex hormones decreases below the initial level: in men, the concentration of testosterone reaches the post-castration level, in women the concentration of estradiol decreases to a level corresponding to oophorectomy or postmenopause. This condition persists throughout the entire period of treatment, which leads to growth inhibition and reverse development of hormone-dependent tumors (uterine fibroids, prostate cancer). After cessation of treatment, physiological hormone secretion is restored.
Pharmacokinetics: Inactive when taken orally. The bioavailability of the drug with subcutaneous and intramuscular administration is comparable. Bioavailability in men is 98%, in women – 75%. The average equilibrium volume of distribution is 27 l. Bonding with plasma proteins is 43-49%. System clearance - 7.6 l. Leuprorelin, being a peptide, is subject to metabolic degradation, mainly by peptidase. The main metabolite is MI. TCmax of the MI metabolite is 2-6 hours and corresponds to 6% of 656 ng/ml. 1 week after injection, the average concentration of MI in plasma is 20% of the average concentration of leuprorelin. 3 hours after a single administration of 11.25 mg (depot form, effective for 3 months), the Cmax of leuprorelin in plasma is 21.82 ± 11.24 ng/ml. Css is achieved 7-14 days after injection. By 4 weeks, the average concentration of leuprorelin in plasma is 0.26±0.1 ng/ml, by 12 weeks the concentration decreases to 0.17±0.08 ng/mg. It circulates in plasma in small but detectable quantities for more than 30 days after the last administration. T1/2 - 3 hours. Leuprorelin and its metabolite MI are excreted by the kidneys - less than 5% of the administered dose within 27 days after injection.
Special instructions: In women of reproductive age, possible pregnancy must be excluded before starting treatment, and non-hormonal methods of contraception should be used during therapy. When treating prostate cancer, antiandrogens should be prescribed to prevent symptoms associated with increased plasma testosterone concentrations. During the treatment period, it is necessary to monitor the activity of LDH and “liver” transaminases, and before starting a second course, it is necessary to determine the bone density (the drug increases the risk of developing osteoporosis). With endometriosis, after completing 6 months of therapy, menstruation resumes after 3 months. During the treatment period, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Drug registration number: P No. 015554/01
Date of registration (re-registration) of the drug: 04/12/2004