Erase capsules 10 mg No. 30
Compound
Each capsule contains isotretinoin 10 mg.
Pharmacokinetics
Retinoid for systemic treatment of acne
Isotretinoin is a stereoisomer of all-trans retinoic acid (tretinoin). The exact mechanism of action of Sotret has not yet been clarified, but it has been established that the improvement in the clinical picture of severe forms of acne is associated with suppression of the activity of the sebaceous glands and a histologically confirmed reduction in their size. In addition, isotretinoin has been shown to have anti-inflammatory effects on the skin.
Hyperkeratosis of epithelial cells of the hair follicle and sebaceous gland leads to desquamation of corneocytes into the gland duct and to blockage of the latter with keratin and excess sebaceous secretion. This is followed by the formation of comedones and, in some cases, the addition of an inflammatory process. Erase suppresses the proliferation of sebocytes and acts on acne, restoring the normal process of cell differentiation. Sebum is the main substrate for the growth of Propionibacterium acnes, so reducing sebum production inhibits bacterial colonization of the duct.
Indications for use
Severe forms of acne (nodulocystic, conglobate acne or acne with risk of scarring). Acne that does not respond to other types of therapy.
Contraindications
— Pregnancy, breastfeeding — liver failure, hypervitaminosis A, severe hyperlipidemia, concomitant therapy with tetracyclines. - Hypersensitivity to the drug or its components. — Children's age up to 12 years. With caution: history of depression, diabetes mellitus, obesity, lipid metabolism disorders, alcoholism.
Directions for use and doses
Standard dosage regimen: Orally, during meals, once or twice a day.
The therapeutic effectiveness of Sotret and its side effects depend on the dose and vary among different patients. This dictates the need for individual dose selection during treatment.
Treatment with Sotret should begin with a dose of 0.5 mg/kg per day. In most patients, the dose ranges from 0.5 to 1.0 mg/kg body weight per day. Patients with very severe forms of the disease or with acne of the trunk may require higher daily doses - up to 2.0 mg/kg. It has been proven that the frequency of remission and prevention of relapses are optimal when using a course dose of 120-150 mg/kg (per course of treatment), therefore the duration of therapy in specific patients varies depending on the daily dose. Complete remission of acne can often be achieved within 16-24 weeks of treatment. In patients who tolerate the recommended dose very poorly, treatment can be continued at a lower dose, but last longer.
In most patients, acne completely disappears after a single course of treatment. In case of obvious relapse, a second course of treatment with Sotret is indicated in the same daily and course dose as the first. Since improvement can continue up to 8 weeks after discontinuation of the drug, a second course should be prescribed no earlier than the end of this period.
Dosing in special cases
In patients with severe renal impairment, treatment should be initiated at a lower dose (eg, 10 mg/day) and further increased to 1 mg/kg/day or the maximum tolerated.
Storage conditions
In a place protected from light and moisture, at a temperature not exceeding 25 C
Best before date
2 years
special instructions
Sotret should be prescribed only by physicians, preferably dermatologists, who have experience in the use of systemic retinoids and are aware of the risk of teratogenicity of the drug. To avoid accidental effects of the drug on the body of other people, donated blood should not be taken from patients who are receiving or who have recently (1 month) received Sotret.
It is recommended to monitor liver function and liver enzymes before treatment, 1 month after treatment, and then every 3 months or as indicated. A transient and reversible increase in liver transaminases was noted, in most cases within normal values. If the level of liver transaminases exceeds the norm, it is necessary to reduce the dose of the drug or discontinue it. Fasting serum lipid levels should also be determined before treatment, 1 month after initiation, and then every 3 months or as indicated. Typically, lipid concentrations normalize after dose reduction or discontinuation of the drug, as well as with diet. It is necessary to monitor a clinically significant increase in triglyceride levels, since their rise above 800 mg/dL or 9 mmol/L can be accompanied by the development of acute pancreatitis, possibly fatal. In case of persistent hypertriglyceridemia or symptoms of pancreatitis, Sotret should be discontinued. In rare cases, depression, psychotic symptoms, and very rarely, suicidal attempts have been described in patients treated with Sotret. Although their causal relationship with the use of the drug has not been established, special caution should be exercised in patients with a history of depression and all patients should be monitored for the occurrence of depression during treatment with the drug, if necessary, referring them to an appropriate specialist. However, discontinuation of Sotret may not lead to the disappearance of symptoms and further observation and treatment by a specialist may be required. In rare cases, at the beginning of therapy, an exacerbation of acne is observed, which resolves within 7-10 days without adjusting the dose of the drug.
Several years after the use of Sotret for the treatment of dyskeratosis, at a total course dose and duration of therapy higher than those recommended for the treatment of acne, bone changes developed, including premature closure of the epiphyseal growth plates, hyperostosis, calcification of ligaments and tendons. Therefore, when prescribing the drug to any patient, the ratio of possible benefits and risks should first be carefully assessed.
Patients receiving Sotret are recommended to use moisturizing ointment or body cream, lip balm to reduce dry skin and mucous membranes at the beginning of therapy. While taking Sotret, pain in muscles and joints, an increase in serum creatinine phosphokinase, which may be accompanied by a decrease in tolerance to intense physical activity, are possible.
Deep chemical dermoabrasion and laser treatment should be avoided in patients receiving Sotret, as well as for 5-6 months after the end of treatment due to the possibility of increased scarring in atypical places and the occurrence of hyper- and hypopigmentation. During treatment with Sotret and for 6 months after it, hair removal using wax applications cannot be performed due to the risk of epidermal detachment, scar development and dermatitis.
Since some patients may experience a decrease in night vision acuity, which sometimes persists even after the end of therapy, patients should be informed about the possibility of this condition, advising them to exercise caution when driving at night. Visual acuity must be carefully monitored. Dryness of the conjunctiva of the eyes, corneal opacities, deterioration of night vision and keratitis usually disappear after discontinuation of the drug. If the mucous membrane of the eyes is dry, you can use applications of a moisturizing eye ointment or an artificial tear preparation. Patients with dry conjunctiva should be monitored for possible development of keratitis. Patients with vision complaints should be referred to an ophthalmologist and consider the advisability of discontinuing Sotret. If you are intolerant to contact lenses, you should use glasses during therapy. Exposure to sunlight and UV rays should be limited. If necessary, use sunscreen with a high protection factor of at least 15 SPF.
Rare cases of the development of benign intracranial hypertension (pseudo brain tumor) have been described, incl. when combined with tetracyclines. In such patients, Sotret should be discontinued immediately. During therapy with Sotret, inflammatory bowel disease may occur. In patients with severe hemorrhagic diarrhea, Sotret must be discontinued immediately.
Rare cases of anaphylactic reactions that occurred only after previous external use of retinoids have been described. Severe allergic reactions dictate the need to discontinue the drug and carefully monitor the patient. Patients at high risk (with diabetes, obesity, chronic alcoholism or lipid metabolism disorders) may require more frequent laboratory monitoring of glucose and lipid levels when treated with Sotret. If diabetes is present or suspected, more frequent monitoring of glycemia is recommended.
Side effects
Most of the side effects of Sotret depend on the dose. As a rule, when prescribing the recommended doses, the benefit-risk ratio, taking into account the severity of the disease, is acceptable for the patient. Side effects are usually reversible after dose adjustment or drug discontinuation, but some may persist after treatment is stopped.
Symptoms associated with hypervitaminosis A: dry skin, mucous membranes, incl. lips (cheilitis), nasal cavity (bleeding), hypopharynx (hoarseness), eyes (conjunctivitis, reversible corneal opacity and contact lens intolerance). Skin and its appendages, rash, itching, facial erythema/dermatitis, sweating, pyogenic granuloma, paronychia, onychodystrophy, increased proliferation of granulation tissue, persistent hair thinning, reversible hair loss, fulminant forms of acne, hirsutism, hyperpigmentation, photo sensitization, photoallergy, mild skin injury. At the beginning of treatment, acne may worsen and persist for several weeks. Musculoskeletal system: muscle pain with or without increased serum CPK levels, joint pain, hyperostosis, arthritis, calcification of ligaments and tendons, other bone changes, tendinitis. Central nervous system and mental sphere: behavioral disturbances, depression, headache, increased intracranial pressure (pseudo-brain tumor: headache, nausea, vomiting, blurred vision, papilledema), seizures. Sense organs: isolated cases of impaired visual acuity, photophobia, impaired dark adaptation (reduced twilight visual acuity), rarely - impaired color vision (passing after drug withdrawal), lenticular cataract, keratitis, blepharitis, conjunctivitis, eye irritation, papilledema (as a manifestation intracranial hypertension), hearing impairment at certain sound frequencies. Gastrointestinal tract, nausea, diarrhea, inflammatory bowel diseases (colitis, ileitis), bleeding, pancreatitis (especially with concomitant hypertriglyceridemia above 800 mg/dl). Rare cases of pancreatitis with a fatal outcome have been described. Transient and reversible increase in the activity of liver transaminases, isolated cases of hepatitis. In many of these cases, the changes did not go beyond the normal range and returned to the initial values during treatment, but in some situations there was a need to reduce the dose or cancel Sotret. Respiratory organs: rarely - bronchospasm (more often in patients with a history of bronchial asthma). Blood system: anemia, decreased hematocrit, leukopenia, neutropenia, increased or decreased platelet count, accelerated ESR. Laboratory indicators: hypertriglyceridemia, hypercholesterolemia, hyperuricemia, decreased levels of high-density lipoproteins, rarely hyperglycemia. While taking Sotret, cases of newly diagnosed diabetes mellitus were reported. In some patients, especially those involved in intense physical activity, isolated cases of increased CK activity in the serum have been described. Immune system: local or systemic infections caused by gram-positive pathogens (Staphylococcus aureus). Other: lymphadenopathy, hematuria, proteinuria, vasculitis (Wegener's granulomatosis, allergic vasculitis), systemic hypersensitivity reactions, glomerulonephritis.
Use during pregnancy and breastfeeding
Pregnancy is an absolute contraindication for Sotret therapy. If pregnancy occurs, despite warnings, during treatment or within a month after the end of therapy, there is a very high risk of giving birth to a child with severe malformations.
Isotretinoin is a drug with a strong teratogenic effect. If pregnancy occurs during a period when a woman takes isotretinoin orally (at any dose and even for a short time), there is a very high risk of giving birth to a child with developmental defects. Sotret is contraindicated in women of childbearing age, unless the woman’s condition meets all of the following criteria:
she must suffer from a severe form of acne that is resistant to conventional treatments, she must clearly understand and follow the doctor’s instructions, she must be informed by the doctor about the danger of pregnancy during treatment with Sotret, within one month after it and urgent consultation if pregnancy is suspected , she must be warned about the possible ineffectiveness of contraception, she must confirm that she understands the essence of the precautions, she must understand the need and continuously use effective methods of contraception for one month before treatment with Sotret, during treatment and for a month after its end ( see section Interaction with other medicinal products), it is advisable to use 2 different methods of contraception at the same time, including barrier, she must have a negative result of a reliable pregnancy test within 11 days before starting the drug, a pregnancy test is strongly recommended to be carried out monthly during treatment and 5 weeks after the end of therapy, she should begin treatment with Sotret only on the 2-3rd day of the next normal menstrual cycle, she must understand the need to visit a doctor every month, when treating for relapse of the disease, she must constantly use the same effective methods of contraception within one month before starting treatment with Sotret, during treatment and for a month after its completion, as well as undergoing the same reliable pregnancy test, she must fully understand the need for precautions and confirm her understanding and desire to use reliable methods of contraception that The doctor explained to her.
Use of contraception as directed above during treatment with isotretinoin should be recommended even in women who do not routinely use contraception due to infertility (except in patients who have had a hysterectomy), amenorrhea, or who report not being sexually active.
The doctor must be sure that: the patient suffers from severe acne (nodular cystic acne, conglobate acne or acne with risk of scarring), acne that is not amenable to other types of therapy, a negative result of a reliable pregnancy test was obtained before taking the drug, during therapy and 5 weeks after the end of therapy, the dates and results of the pregnancy test must be documented, the patient uses at least 1, preferably 2 effective methods of contraception, including a barrier method, for one month before starting treatment with Sotret, during treatment and during the month after its completion, the patient is able to understand and fulfill all of the above requirements for pregnancy protection, the patient meets all of the above conditions.
Pregnancy test
According to current practice, a pregnancy test with a minimum sensitivity of 25 tIU/ml should be performed in the first 3 days of the menstrual cycle:
Before starting therapy: To exclude possible pregnancy, the result and date of the initial pregnancy test must be recorded by a doctor before starting contraception. In patients with irregular menstruation, the timing of a pregnancy test depends on sexual activity and should be performed 3 weeks after unprotected intercourse. The doctor should inform the patient about contraceptive methods. A pregnancy test is carried out on the day of Sotret's prescription or 3 days before the patient's visit to the doctor. The specialist should record the test results. The drug can only be prescribed to patients receiving effective contraception for at least 1 month before starting Sotret therapy.
During therapy: The patient must visit the doctor every 28 days. The need for monthly pregnancy testing is determined in accordance with local practice and taking into account sexual activity and previous menstrual irregularities. If indicated, a pregnancy test is performed on the day of the visit or three days before the visit to the doctor, the test results must be recorded.
End of therapy: 5 weeks after the end of therapy, a test is performed to exclude pregnancy.
A prescription for Sotret for a woman capable of childbearing can be issued only for 30 days of treatment; continuation of therapy requires a new prescription of the drug by a doctor. It is recommended that a pregnancy test, writing a prescription and receiving the drug be carried out on the same day. Sotret should be dispensed in a pharmacy only within 7 days from the date of issuing the prescription. Full information about teratogenic risk and strict adherence to measures to prevent pregnancy should be provided to both men and women.
Interaction
Due to the possible increase in symptoms of hypervitaminosis A, the simultaneous administration of Sotret and vitamin A should be avoided. Since tetracyclines can also cause an increase in intracranial pressure, their use in combination with Sotret is contraindicated. Isotretinoin may reduce the effectiveness of progesterone preparations, so you should not use contraceptives containing low doses of progesterone. Combined use with topical keratolytic or exfoliative drugs for the treatment of acne is contraindicated due to the possible increase in local irritation.
Overdose
In case of overdose, signs of hypervitaminosis A may appear. In the first few hours after an overdose, gastric lavage may be necessary.
Introduction
Acne (acnevulgaris) or acne vulgaris is one of the most common skin diseases in Europe [1] and the most common in the United States [2], affecting more than 80% of adolescents and young adults [1].
In many cases, acne can last for decades [3]. This is a chronic inflammatory disease of the pilosebaceous apparatus, manifested by erythema, edema, discomfort, and scar formation in 43% of patients [4]. As is common with dermatological conditions [5], such symptoms often have psychological and social consequences [6].
Treatment varies depending on the severity, from topical therapy (retinoids, benzoyl peroxide, antibiotics) in mild cases to oral antibiotics, hormonal treatment, and oral isotretinoin in moderate to severe cases.
Current European consensus documents [7] strongly recommend oral isotretinoin for moderate to severe papulopustular/nodular acne at 0.3–0.5 mg/kg/day and acne conglobata at ≥ 0.5 mg/kg/day . Systemic therapy, including isotretinoin, is generally not recommended for mild to moderate papulopustular acne [7,8].
The reported side effects of oral isotretinoin [9,10] have been of concern and are likely to have influenced prescribing patterns. Although subsequent studies and reviews have shown a good safety profile [11], there may still be persistent reluctance to prescribe oral isotretinoin.
Delay in administering adequate treatment can lead to scar formation, which will affect quality of life. There should be no undertreatment of this widespread condition.
We describe our experience with the use of oral isotretinoin in patients with acne vulgaris, drawing on relevant publications covering key points associated with the use of isotretinoin, namely: comparison of oral isotretinoin use with antibiotics, psychological aspects in acne and oral isotretinoin treatment, side effects of oral isotretinoin treatment and aesthetic approaches that can alleviate predictable side effects.
Using isotretinoin or taking antibiotics
Acne treatment must take into account all factors: abnormal keratinization, sebum production, bacterial flora and especially inflammation. Patients should be offered a simple treatment regimen initially to increase adherence [12].
Oral antibiotics are commonly used to treat moderate to severe acne; they should be used in conjunction with topical treatments [13], preferably containing benzoyl peroxide [14].
In recent years, the exponential increase in antibiotic resistance has led to restrictions on antibiotic use—oral antibiotics should be used for no more than 3 months [15].
Accordingly, alternative methods are required [16]. One alternative is oral isotretinoin [17].
According to Peck, oral isotretinoin was effective in the treatment of severe forms of acne and resulted in complete recovery in 13 of 14 patients treated [18].
- The main indications for the use of oral isotretinoin are:
- Severe nodular cystic acne
- Severe papulopustular acne.
- Nodular rashes of moderate to severe severity.
- Insufficient effect on previous treatment.
- Tendency to form scars.
- Sudden relapse after cessation of therapy.
- Patients experiencing severe psychological stress.
More recently, the range of use of oral isotretinoin has been expanded to include mild inflammatory acne that has resulted in scarring or when the patient experiences significant psychological distress. Low doses of isotretinoin are effective against hyperseborrhea and mild inflammatory acne that do not respond to systemic antibiotics due to acquired resistance. Early recognition of non-response to antibiotics is important [19].
Nagler and colleagues conducted a clinical trial at New York University in 137 patients treated with oral isotretinoin between 2005 and 2014, examining systemic antibiotic use in these patients before isotretinoin treatment.
They reported that the mean duration of antibiotic therapy was 331.3 days; 15% of antibiotics were prescribed for 3 months or less, 64% for 6 months or more, and 34% for 1 year or more. The study concluded that early identification of patients who fail to respond to oral antibiotics is important.
It is also strongly recommended that you decide to stop taking antibiotics within 6-8 weeks and consider using isotretinoin.
Our experience and recommendations
For 30 years, we (Piquero et al.[21]) have used isotretinoin at low doses or 0.2–0.3 mg/kg/day for 12 months followed by topical maintenance therapy with excellent response in over 80 % cases with moderate inflammatory acne in patients prone to scar formation.
For severe inflammatory acne, we continue to use the doses recommended in international consensus documents. Based on the literature and our experience, patients should be offered a simple treatment regimen to improve adherence.
The indications for the use of systemic antibiotics and the duration of their use should be limited, especially when effective alternatives are available [22].
Psychological aspects of acne and the role of oral isotretinoin
Acne has a significant impact on the psychological state of most patients with this disease. Mainly in the form of depressive symptoms [23,24].
Oral isotretinoin provides the most effective therapeutic effect for acne, significantly improving symptoms. However, a study published in 1983 stated that oral isotretinoin may cause symptoms of depression [9].
Since then, numerous publications have debated this issue. Those who have found a positive association between isotretinoin and depression have concluded that this is due to an idiosyncratic effect observed in those patients who have a high family history of depression [25,26].
However, most studies have not found an association between oral isotretinoin and depression, but rather have found a positive effect of treatment on reducing depressive symptoms [27–32].
A systematic review and meta-analysis examining the association between oral isotretinoin and depression was published in 2021 [33].
It included 31 controlled or prospective uncontrolled studies involving ≥ 15 patients treated with oral isotretinoin for acne. In total, the work assessed data on 2939 patients. Scores were calculated according to the main depression scales before and at the end of treatment.
The data showed no significant increase in depressive symptoms in patients receiving isotretinoin compared with patients receiving other treatments. Rather, scores close to indicating depression were significantly reduced in patients receiving isotretinoin.
However, a decrease in depression scores was noted from the start of treatment and became low after completion of treatment with oral isotretinoin.
In 2004, we (Guerra-Tapia et al [34]) developed a treatment satisfaction questionnaire that included assessments of depression and anxiety scales before, during and after isotretinoin treatment. Approximately 4,000 patients completed the survey.
All respondents reported significant improvement in psychological status, indicating high compliance with oral isotretinoin treatment. All respondents stated that they would be willing to repeat the course of treatment if necessary in the future.
Our experience and recommendations
Treatment of acne with oral isotretinoin does not appear to be associated with increased depression in most patients. On the contrary, depressive symptoms may improve with isotretinoin therapy [11].
There is only a small proportion of patients who experience increased depression and suicidal thoughts while taking this medication. Given this fact and the increased predisposition of adolescents to depressive conditions, it would be prudent to recommend careful monitoring of all patients with acne to identify those who, due to individual predisposition, are at increased risk of developing depression.
Side effects of oral isotretinoin
Isotretinoin is a physiologically active metabolite of vitamin A (retinol) [35].
Almost all patients treated with oral isotretinoin have a good therapeutic outcome [7,9]; however, it may also have side effects. Most of them, with the exception of teratogenicity, are dose dependent.
A recent Cochrane review [36,31] and a randomized controlled trial of 3836 participants concluded that non-serious side effects were more common with isotretinoin than with oral topical antibiotics, but did not draw any conclusions about serious adverse events. Oral isotretinoin should only be used under the supervision of physicians experienced in the use of systemic retinoids in the treatment of acne and with a thorough understanding of the risks of therapy and monitoring requirements.
Patients may also consult with physicians during treatment with isotretinoin, so patient awareness of possible associated side effects is an important factor in adherence.
Adverse effects can be divided into the following categories: 1) teratogenic, 2) clinical: cutaneous or extracutaneous, and 3) laboratory results.
Oral isotretinoin has also been associated with nonspecific side effects affecting various body systems, but these are usually sporadic cases, the clinical significance of which appears to be negligible compared to the sum of all isotretinoin exposures. In addition, it is likely that there is a tendency to overestimate the frequency and severity of side effects in leaflets, as is often the case with other drugs [37].
Teratogenicity
This is the most important side effect [38].
Ingestion of isotretinoin by women of childbearing potential, regardless of dose, can cause serious fetal malformations, premature birth, or spontaneous abortion in a high percentage of cases.
Pregnancy prevention programs (PPPs) in their various forms [39,40] have generally been successful, since unintended pregnancies during treatment with oral isotretinoin, although occurring in small numbers. Female patients undergo laboratory pregnancy testing before starting treatment, then monthly at the beginning of each menstrual cycle during treatment and one month after treatment ends.
They receive detailed oral and written information about isotretinoin treatment in general and contraceptive methods in particular. The use of contraception should begin one month before the start of treatment and continue throughout treatment and one month after stopping treatment. Unfortunately, there is evidence of unsuccessful and ineffective PPPs[41].
Therefore, it is critical that patients receive accurate information and stay in touch with their doctor. In addition, two recent articles highlight unintended circumstances that may result in delayed initiation of therapy or premature discontinuation of treatment, especially in certain populations [42,43].
In 2021, the European Medicines Agency published a review on systemic retinoids, highlighting the need for updated pregnancy prevention measures [44].
Skin side effects
Side effects such as dry skin and mucous membranes are very common. Moreover, their absence may raise the question of insufficient dosage. Xerosis occurs secondary to pharmacologically induced sebum suppression [45] and epidermal dyscohesion (?) [4,6,47].
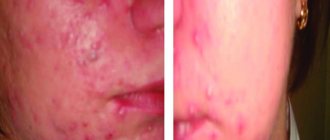
The most common clinical side effect is cheilitis (Fig. 1), dry lips, which can vary from xerosis to painful fissures [48].
The degree of skin dryness and flaking depends on the dose. These symptoms are often observed in areas with the largest number of sebaceous glands, namely the face, chest and back. Dryness of the mucous membranes of the nose and eyes may also occur when taking too high doses. Severe skin reactions (eg, erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis) have been observed very rarely [49].
Some patients experience initial worsening of acne in the first month of treatment; less commonly, isotretinoin can cause fulminant acne [50]. This is likely due to the dose of isotretinoin being too high for this patient.
Extradermal side effects
During treatment, headache, fatigue and visual disturbances may occur, including night vision disturbances, keratitis and corneal opacities. These complications may be dose-dependent. Benign intracranial hypertension has been reported when isotretinoin is used together with certain antibiotics [51].
Gastrointestinal disorders associated with colitis and inflammatory bowel disease may also occur during isotretinoin treatment, but a precise cause-and-effect relationship has not been established [52,53].
Indeed, meta-analyses found no association between an increased risk of IBD and isotretinoin use [54]. Psychiatric side effects of oral isotretinoin are discussed elsewhere in this article.
Changes in laboratory tests
Lipids: Oral isotretinoin may cause a dose-dependent increase in blood cholesterol and triglyceride levels. A large meta-analysis [53] on this topic found that oral isotretinoin was associated with a statistically significant change in the mean of these parameters, but the mean increase did not meet high-risk criteria.
In addition, the proportion of patients with such laboratory abnormalities was low enough that it was concluded that monthly testing (as indicated in the drug instructions) was not necessary. Laboratory tests are recommended before starting treatment, after 6 weeks, and then every 3 months during therapy [56].
If hypertriglyceridemia cannot be controlled, oral isotretinoin should be discontinued.
Liver transaminases may also increase in a dose-dependent manner during treatment. It should be kept in mind that there are many possible causes of elevated transaminase levels and these should be ruled out. In a recent review, the increase in transaminases due to oral isotretinoin was determined to be transient and usually not requiring discontinuation of treatment [57].
Transaminases should be checked before treatment, after 6 weeks, and then every 3 months (early testing is required only if clinically necessary).
Creatinine phosphokinase (CPK) is a muscle enzyme whose levels physiologically increase during exercise. Oral isotretinoin is considered another possible inducer of this enzyme (dose-dependent).
There are few clinical studies on the relationship between CPK and oral isotretinoin, and most published articles are only case reports [58]. The prevalence of elevated CPK levels ranges from 10% to 50% [58].
However, this fact must be assessed individually for each patient. Moderate exercise is a known cause of elevated CPK levels.
Muscle symptoms are sometimes reported, but evidence of muscle damage is very rarely found [10,58].
Rare cases of bone changes have been observed, but these have been associated with very high dosage of isotretinoin, long duration of treatment and overall high cumulative dose [59–62].
Monitoring for abnormalities in CBC parameters is not of great importance since abnormalities occur infrequently and are unlikely to affect treatment [63].
Our experience and recommendations
The correct dosage is critical when taking oral isotretinoin. Each patient has individual characteristics regarding drug absorption, bioavailability and side effects.
In addition, dermatologists should be aware of differences in the production of the active substance and the widespread availability of oral isotretinoin analogues. The literature shows that a low starting daily dose of 0.1–0.2 mg/kg/day or about 10 mg per day and gradually increasing to the maximum tolerated dose is the best way to obtain good clinical results while minimizing side effects compared with the standard dose 0.5 mg/kg/day [64].
All medications can cause side effects. Obviously, the balance between clinical effectiveness and side effects must be tilted towards the former.
A review of the effectiveness and side effects of oral isotretinoin, including only randomized control trials involving 760 patients, found that only 12 patients discontinued treatment due to side effects [49].
Oral isotretinoin is undoubtedly the most effective drug for treating severe forms of acne that do not respond to other treatments.
It is sometimes incorrectly described as a dangerous drug, without pointing out that the only real concern is teratogenicity in patients of childbearing age.
Cosmetological aspects
Cosmetic treatment of acne is of great importance and should also be recommended to patients [65]. Naturally, patients want to get good therapeutic results in the shortest possible time.
Oral isotretinoin, which is so useful in the treatment of acne, affects the skin barrier function, lipids, sebaceous glands and skin microflora. Cosmetic treatments and general recommendations help reduce side effects, thereby promoting adherence to treatment, which contributes to better therapeutic efficacy and a better quality of life for patients [66].
In dermatology in general and especially in acne, dermatocosmetics are very important. Dermatocosmetics helps to minimize the side effects of pharmacological treatment [67].
Numerous studies have shown that adherence to acne medications is particularly low when a person is prescribed a combination of topical and systemic treatments [3,68].
It is important to begin using dermocosmetics early in treatment with oral isotretinoin to minimize side effects and improve adherence. Dermatocosmetics should be selected according to their constituent ingredients to suit the specific needs of the patient.
Oral isotretinoin is associated with the following structural and functional changes in the skin: [46,69]
Modified barrier function
Xerosis, fissures and eczema are common side effects associated with impaired skin barrier function. As a consequence, there is an imbalance between moisture loss and retention (permeability barrier), decreased recognition and neutralization of microbes (antimicrobial barrier), and loss of the antioxidant barrier.
Consequently, there is an increased risk of sun exposure (sunscreen barrier) and altered response to exogenous allergens and haptens (immune barrier) [46].
Corneocyte decohesion
Oral isotretinoin causes increased epidermal turnover and “fragility” of the skin, which makes it sensitive to intradermal “separation”.
There is a loss of contact between desmosomes and a decrease in the number of tonofibrils. Corneocytes are easily separated from the outer part of the stratum corneum, which explains the desquamation that patients experience.
In addition, depending on the dose of isotretinoin, transepidermal water loss (TEWL) increases [70]. These changes trigger a cycle of inflammation, the release of inflammatory cytokines, which contribute to hyperproliferation of the epidermis and the appearance of hyperkeratosis [46,71].
In 2007, we (Herane et al) conducted a prospective, double-blind, placebo-controlled study [72] of adjuvant therapy with oral isotretinoin (0.4–0.7 mg/kg) using a specific moisturizing gel cream containing the active substances , including biosaccharide gum(?) (from the English biosaccharide gum-2), hyaluronic acid and glycerin.
After one month of treatment, compared with the placebo group, the adjuvant group had 19% higher hydration levels and lower TEWL (barrier effect) levels than the isotretinoin-only group.
Changes in lipid composition
It is important to distinguish between lipids that are physiologically integrated into the intercellular lipid membrane (ILM) and sebum-derived lipids that are secreted in the follicular duct and “flow” to the skin surface. Studies in mice have shown that isotretinoin does not alter ILM [46,70].
The sebum suppression effect of oral isotretinoin (at the recommended total dose of 100–120 mg/kg) begins after one month of treatment. The reverse effect begins approximately 4-5 months after completion of treatment.
At week 6 of treatment with oral isotretinoin, a change in lipid composition begins with a decrease in the proportion of glycerides (by 36%), an increase in free stearins (34%) and total ceramides (19%), as well as an increase in the ratio of free stearins: in particular cholesterol (86%) [ 73].
Cholesterol increases by 10–25%, and the proportion of waxy esters and squalene decreases [45,46]. This leads to a decrease in comedogenesis and the number of comedones [71,74].
Skin microflora
Oral isotretinoin causes changes in the type and number of microorganisms that colonize the skin and nasal mucosa, with increased colonization by Staphylococcus aureus and a marked decrease in the number of Propionibacterium acnes from the beginning of treatment, and which continues for some time after the end of treatment [75].
Our experience and recommendations
Appropriate care for acne-prone skin while taking retinoids includes gentle cleansing, restoring the skin barrier, and maintaining barrier function [72].
For cleansing, you should use soft special products, cleansing emulsions or micellar lotions [66].
The use of abrasive cleansers and products containing sulfur, glycolic acid or similar substances should be avoided. Moisturizers and emollients should be non-comedogenic, hypoallergenic, non-irritating, non-greasy, and water-based [76].
It is very important to use special moisturizers that contain active ingredients of high quality and concentration to protect and restore the skin. Products with hyaluronic acid, glycerin, biosaccharides, oligosaccharides, etc. are suitable. [72,77].
During periods of high sun exposure, especially in countries with a thinning ozone layer, dermatitis, eczema, cheilitis and other signs of sun sensitivity often occur on areas exposed to light: mainly the face and hands.
In this case, you should use an SPF 50+ UVA/UVB sunscreen that has been specifically tested to be comedogenic for oily, acne-prone skin. E
It must be applied every 2 hours, as well as after swimming or after heavy sweating. For lips and mucous membranes, it is recommended to use Vaseline at night and lip balms with an SPF 30–50 filter several times a day.
Eye drops and gels are especially useful for patients who wear contact lenses or live in areas with high levels of air pollution. You should also wear sunglasses at night, especially in the summer and when participating in any outdoor activities.
When it comes to hair removal, cold wax or razors are preferable to hot wax or lasers, followed by moisturizer and sun protection. Hair removal using intense pulsed light (IPL) must be done with caution.
There is controversy regarding procedures such as dermabrasion, chemical abrasives, CO2 laser, and fractional radiotherapy.
Some practitioners delay such procedures for more than 6 months [55] after stopping oral isotretinoin, while others wait 20 to 30 days (the time it takes for the drug to clear).
Two recent reviews [78,79] do not recommend mechanical dermabrasion and ablative laser [78].
However, there is still insufficient evidence to support delaying microdermabrasion, superficial chemical peels, fractional ablative and fractional non-ablative laser resurfacing for patients who are currently taking or have recently taken isotretinoin (6–12 months) [78,79].
Our current practice is to wait approximately 2 months after stopping isotretinoin before starting such procedures. Thus, the use of special cosmetics increases treatment adherence by 2.2 times with a noticeable reduction in side effects [66].
conclusions
Acne significantly affects the psychological state of patients, often manifesting itself in the form of depressive symptoms. Timely effective treatment not only helps to minimize the risk of permanent scarring, but also helps with psychological aspects.
Therefore, there is an improvement in the patient’s quality of life. Appropriate cosmetic treatment should be considered alongside primary pharmacological treatment to reduce side effects and promote treatment adherence, thereby showing improved results.
Oral isotretinoin is an important part of the treatment of acne. This drug has truly changed the lives of patients.
The most serious side effect is teratogenicity, so compliance with existing pregnancy prevention programs is of paramount importance, regardless of isotretinoin dosage.
Other side effects can be easily prevented and/or treated in the vast majority of cases. Oral isotretinoin, when used correctly, can be considered a reasonable alternative to antibiotics.