Home | About us | Delivery | Advertisers | Login | Registration
The pharmacy is closed on Sundays and holidays.
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2021 Pharmacy 84.
Instructions for use EUPHYLLINE solution
Aminophylline has a narrow therapeutic index. Theophylline toxicity is most likely to occur at serum concentrations greater than 20 mcg/mL and becomes increasingly severe at higher serum concentrations.
Doses greater than 3 g may be serious in adults (40 mg/kg in a child). The lethal dose may be as low as 4.5 g in adults (60 mg/kg in children), but is usually higher.
Death in adults may occur when aminophylline is administered IV in large doses in patients with renal, hepatic or cardiovascular impairment, or if the injection is given quickly.
Symptoms:
tachycardia, in the absence of hypoxia, fever, or during coadministration of sympathomimetic drugs, may be a sign of theophylline toxicity.
Gastrointestinal symptoms:
anorexia, nausea, vomiting, diarrhea, vomiting blood.
Neurological symptoms:
restlessness, insomnia, irritability, headache, agitation, hallucinations, extreme thirst, slight fever, dilated pupils and tinnitus. Seizures can occur even without previous symptoms of toxicity and often lead to death. Coma can develop in very severe cases.
Cardiovascular symptoms:
palpitations, arrhythmias, arterial hypotension, supraventricular and ventricular arrhythmias.
Metabolic symptoms:
hypokalemia can develop quickly and can be serious. Hyperglycemia, albuminuria, hyperthermia, hypomagnesemia, hypophosphatemia, hypercalcemia, respiratory alkalosis, metabolic acidosis, and rhabdomyolysis may also occur.
Treatment:
Treatment of overdose is supportive and symptomatic.
Serum theophylline and potassium levels should be checked. Repeated oral administration of activated charcoal helps eliminate theophylline from the body, even after intravenous administration. Aggressive antiemetic therapy may be required to allow oral administration of activated charcoal.
Convulsions can be stopped by intravenous administration of diazepam 0.1-0.3 mg/kg to 10 mg/kg. Restoring fluid and electrolyte balance is essential. Hypokalemia should be corrected by IV infusion of potassium chloride. Sedation with diazepam may be required in agitated patients.
Propranolol can be administered IV to reverse tachycardia, hypokalemia and hyperglycemia, provided the patient does not suffer from asthma.
In general, theophylline is metabolized quickly and hemodialysis is not warranted. In patients with congestive heart failure or liver disease, hemodialysis may increase the clearance of theophylline by 2-fold.
Hemosorption should be considered if:
- intestinal obstruction prevents the administration of several doses of activated carbon:
- plasma theophylline concentrations >80 mg/l (acute) or >60 mg/l (chronic). In the elderly, hemosorption should be considered at theophylline concentrations >40 mg/L. Clinical signs, rather than theophylline concentration, are the best guide for treatment.
Eufillin solution for intravenous administration 24 mg/ml in ampoules 5 ml No. 10
Name
Eufillin solution din. 24 mgml per pack. 5 ml per pack No. 10
Release form
Solution
Description
clear, colorless or slightly yellowish liquid.
Compound
One ampoule (5 ml) contains - active ingredient: aminophylline for injection - 120 mg; excipient: water for injection.
INDICATIONS FOR USE
Broncho-obstructive syndrome in bronchial asthma and chronic obstructive pulmonary disease.
CONTRAINDICATIONS
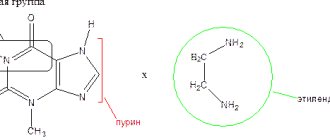
- hypersensitivity to ethylenediamine or allergy to theophyllines, caffeine and theobromine,
- simultaneous use with other xanthine-containing drugs. When therapeutic doses of aminophylline and/or theophylline are administered simultaneously, by more than one route of administration, or in more than one drug product, the risk of serious toxicity increases,
- children up to 6 months old,
- acute porphyria,
- acute period of myocardial infarction,
- paroxysmal tachycardia.
METHOD OF APPLICATION AND DOSES
The drug is intended for slow intravenous administration. The solution should be administered very slowly over 4-6 minutes, 5-10 ml of the drug (0.12-0.24 g), which is pre-diluted with a small volume (5-10 ml) of 5% dextrose or 0.9% solution sodium chloride for injection. Before administration, the solution must be warmed to body temperature. Eufillin is administered parenterally up to 3 times a day, for no more than 14 days. Higher doses of aminophylline for adults when administered intravenously: single - 0.25 g, daily - 0.5 g. Maintenance therapy can be provided by administering large volumes of infusion solutions, the rate of administration is adjusted so as to provide the required amount of the drug every hour. Typically, when administered by drip, 10-20 ml of the drug (0.24-0.48 g) is diluted in 100-150 ml of 0.9% sodium chloride solution and administered at a rate of 30-50 drops per minute. Theophylline therapeutic plasma concentrations are considered to range from 5 to 20 mcg/mL, and levels above 20 mcg/mL are most likely associated with toxicity. There is also individual patient variation in the dosage required to achieve plasma theophylline concentrations within the desired therapeutic range. During therapy, patients should be closely monitored for toxicity and, where possible, theophylline levels should also be monitored; doses should be based on ideal body weight; the drug is not recommended for children under 6 months of age due to significant fluctuations in the metabolism of theophylline in young children. Patients who have not received drugs containing theophylline A. A loading dose of aminophylline 6 mg/kg body weight can be administered intravenously slowly at a rate not exceeding 25 mg/min. B. Depending on the patient's condition, the maintenance dose for the next 12 hours can be calculated as follows:
- children aged 6 months to 9 years: 1.2 mg/kg/hour (decrease to 1 mg/kg/hour after 12 hours);
- children aged 9 to 16 years and young adult smokers: 1 mg/kg/hour (decrease to 0.8 mg/kg/hour after 12 hours);
- healthy non-smoking adults: 0.7 mg/kg/hour (decrease to 0.5 mg/kg/hour after 12 hours);
- elderly patients and those with cor pulmonale: 0.6 mg/kg/hour (decrease to 0.3 mg/kg/hour after 12 hours);
- patients with congestive heart failure or liver disease: 0.5 mg/kg/hour (decrease to 0.1-0.2 mg/kg/hour after 12 hours).
Patients already receiving theophylline The loading dose can be calculated on the basis that each 0.5 mg/kg theophylline administered as a loading dose may result in a 1 mcg/mL increase in serum theophylline concentration. Ideally, administration should be delayed until serum theophylline is determined. If this is not possible and if the clinical situation requires that the drug be administered, then administer 3.1 mg/kg aminophylline (equivalent to 2.5 mg/kg theophylline anhydrous) on the basis that this may result in increased serum theophylline concentrations approximately 5 mcg/ml when administered as a loading dose. In the future, the maintenance dose is recommended to be the same as described above.
PACKAGING AND CONDITIONS OF DISCHARGE FROM PHARMACIES
5 ml in ampoules in packaging No. 10, No. 5x2. By doctor's prescription.
Euphyllin (Euphyllini)
Ephedrine, beta-agonists, caffeine and furosemide enhance the effect of the drug. Increases the likelihood of developing side effects of glucocorticosteroids, mineralocorticosteroids (hypernatremia), and general anesthesia agents (the risk of ventricular arrhythmias increases). In combination with phenobarbital, phenytoin, rifampicin, isoniazid, carbamazepine or sulfinpyrazone, a decrease in the effectiveness of aminophylline is observed, which may require an increase in the doses of the drug. Aminoglutethimide and moracizine, being inducers of microsomal oxidation, increase the clearance of aminophylline, which may require an increase in its dose.
The clearance of the drug is reduced when prescribed in combination with macrolide antibiotics, lincomycin, allopurinol, cimetidine, isoprenaline, beta-blockers, which may require a dose reduction.
Oral estrogen-containing contraceptives, antidiarrheal drugs, intestinal sorbents weaken, and H2-histamine blockers, slow calcium channel blockers, mexiletine enhance the effect (they bind to the cytochrome P450 enzymatic system and change the metabolism of aminophylline). When used in combination with enoxacin and other fluoroquinolones, small doses of ethanol, disulfiram, recombinant interferon alpha, methotrexate, propafenone, thiabendazole, ticlopidine, verapamil, and when vaccinated against influenza, the intensity of action of aminophylline may increase, which may require a reduction in its dose. The drug suppresses the therapeutic effects of lithium carbonate and beta-blockers. The administration of beta-blockers interferes with the bronchodilatory effect of aminophylline and can cause bronchospasm. Eufillin potentiates the effect of diuretics by increasing glomerular filtration and reducing tubular reabsorption. With caution, aminophylline is prescribed simultaneously with anticoagulants, other theophylline or purine derivatives. It is not recommended to use aminophylline with drugs that excite the central nervous system (increases neurotoxicity). The drug cannot be used with dextrose solutions and is incompatible with fructose and levulose solutions. The pH of the solutions to be mixed must be taken into account: pharmaceutically incompatible with acid solutions.
Eufillin, 10 pcs., 5 ml, 24 mg/ml, solution for intravenous administration
Ephedrine, beta-agonists, caffeine and furosemide enhance the effect of the drug. Increases the likelihood of developing side effects of glucocorticosteroids, mineralocorticosteroids (hypernatremia), and general anesthesia agents (the risk of ventricular arrhythmias increases). In combination with phenobarbital, phenytoin, rifampicin, isoniazid, carbamazepine or sulfinpyrazone, a decrease in the effectiveness of aminophylline is observed, which may require an increase in the doses of the drug. Aminoglutethimide and moracizine, being inducers of microsomal oxidation, increase the clearance of aminophylline, which may require an increase in its dose.
The clearance of the drug is reduced when prescribed in combination with macrolide antibiotics, lincomycin, allopurinol, cimetidine, isoprenaline, beta-blockers, which may require a dose reduction.
Oral estrogen-containing contraceptives, antidiarrheal drugs, intestinal sorbents weaken, and H2-histamine blockers, slow calcium channel blockers, mexiletine enhance the effect (they bind to the cytochrome P450 enzymatic system and change the metabolism of aminophylline). When used in combination with enoxacin and other fluoroquinolones, small doses of ethanol, disulfiram, recombinant interferon alpha, methotrexate, propafenone, thiabendazole, ticlopidine, verapamil, and when vaccinated against influenza, the intensity of action of aminophylline may increase, which may require a reduction in its dose.
The drug suppresses the therapeutic effects of lithium carbonate and beta-blockers. The administration of beta-blockers interferes with the bronchodilatory effect of aminophylline and can cause bronchospasm. Eufillin potentiates the effect of diuretics by increasing glomerular filtration and reducing tubular reabsorption.
With caution, aminophylline is prescribed simultaneously with anticoagulants, other theophylline or purine derivatives. It is not recommended to use aminophylline with drugs that excite the central nervous system (increases neurotoxicity). The drug cannot be used with dextrose solutions and is incompatible with fructose and levulose solutions. The pH of the solutions to be mixed must be taken into account: pharmaceutically incompatible with acid solutions.
Contraindications
Eufillin is not a completely safe drug and has a fairly wide list of contraindications for its use:
- Individual hypersensitivity to one or more components of the drug.
- Children under 3 years of age.
- From the cardiovascular system: acute period of myocardial infarction, acute and severe chronic heart failure, atherosclerosis, dissecting aortic aneurysm, hypertrophic cardiomyopathy, high-grade arterial hypertension, and low blood pressure.
- From the digestive tract: exacerbation of gastric or duodenal ulcer, GERD, decompensated liver failure.
- Hormonal disorders: hypothyroidism, thyrotoxicosis.
- From the blood system: coagulation disorders and spontaneous bleeding in the anamnesis.
- Acute infectious diseases, sepsis.
- Severe renal failure.
- Epilepsy.
Eufillin amp 24% 1ml N10 (Dalkhimpharm)
Pharmaceutical group: bronchodilator. Pharmaceutical action: Bronchodilator, methylxanthine derivative; inhibits PDE, increases the accumulation of cAMP in tissues, blocks adenosine (purine) receptors; reduces the flow of Ca2+ through the channels of cell membranes, reduces the contractile activity of smooth muscles. Relaxes the bronchial muscles, stimulates the respiratory center and improves alveolar ventilation, which ultimately leads to a reduction in the severity and frequency of apnea episodes. It has a stimulating effect on the activity of the heart, increases strength and heart rate, increases coronary blood flow and myocardial oxygen demand. Reduces the tone of blood vessels (mainly those of the brain, skin and kidneys). It has a peripheral venodilating effect, reduces pulmonary vascular resistance, and lowers pressure in the pulmonary circulation. Increases renal blood flow and has a moderate diuretic effect. Expands extrahepatic bile ducts. Stabilizes mast cell membranes, inhibits the release of mediators of allergic reactions. Inhibits platelet aggregation (suppresses platelet activating factor and PgE2 alpha), increases the resistance of red blood cells to deformation (improves the rheological properties of blood), reduces thrombus formation and normalizes microcirculation. It has a tocolytic effect, increases the acidity of gastric juice. When used in large doses, it has an epileptogenic effect. Pharmacokinetics: After oral administration, it is quickly and completely absorbed, however, the bioavailability and kinetics of absorption depend on the dosage form of the drug. Bioavailability for tablet forms with rapid release of the active substance and liquid dosage forms is 90-100%. When using prolonged dosage forms, absorption parameters and bioavailability may vary. Food reduces the rate of absorption without affecting its magnitude (large volumes of liquid and proteins speed up the process). The higher the dose taken, the lower the absorption rate. TCmax with intravenous administration of 0.3 g - 15 min, Cmax value - 7 μg/ml. TCmax for conventional dosage forms - 1-2 hours, for extended-release forms - 4-7 hours, for tablets with enteric coating - 5 hours. The volume of distribution is in the range of 0.3-0.7 l/kg (30-70% of the “ideal” weight body), on average 0.45 l/kg. Communication with plasma proteins in adults - 60%, in newborns - 36%, in patients with liver cirrhosis - 36%. Penetrates into breast milk (10% of the dose taken), through the placental barrier (the concentration in the fetal blood serum is slightly higher than in the maternal serum). Aminophylline exhibits bronchodilating properties in concentrations of 10-20 mcg/ml. Concentrations above 20 mg/ml are toxic. The stimulating effect on the respiratory center is realized at a lower content of the drug in the blood - 5-10 mcg/ml. Metabolized at physiological pH values with the release of free theophylline, which is further metabolized in the liver with the participation of several cytochrome P450 isoenzymes. As a result, 1,3-dimethyluric acid (45-55%) is formed, which has pharmacological activity, but is 1-5 times inferior to theophylline. Caffeine is an active metabolite and is formed in small quantities, with the exception of premature newborns and children under 6 months, in whom, due to the extremely long half-life of caffeine, its significant accumulation occurs in the body (up to 30% of that for aminophylline). In children over 3 years of age and in adults, the phenomenon of caffeine accumulation is absent. T1/2 in newborns and children up to 6 months - more than 24 hours; in children over 6 months - 3.7 hours; in adults - 8.7 hours; for “smokers” (20-40 cigarettes per day) - 4-5 hours (after quitting smoking, pharmacokinetics normalize after 3-4 months); in adults with COPD, pulmonary heart disease and pulmonary heart failure - over 24 hours. Excreted by the kidneys. In newborns, about 50% of theophylline is excreted unchanged in the urine versus 10% in adults, which is associated with insufficient activity of liver enzymes.
Eufillin
Bronchodilator, xanthine derivative; inhibits phosphodiesterase, increases the accumulation of cyclic adenosine monophosphate in tissues, blocks adenosine (purine) receptors; reduces the flow of calcium ions through the channels of cell membranes, reduces the contractile activity of smooth muscles. Relaxes the bronchial muscles, increases mucociliary clearance, stimulates contraction of the diaphragm, improves the function of the respiratory and intercostal muscles, stimulates the respiratory center, increases its sensitivity to carbon dioxide and improves alveolar ventilation, which ultimately leads to a decrease in the severity and frequency of apnea episodes. By normalizing respiratory function, it helps saturate the blood with oxygen and reduce the concentration of carbon dioxide. It has a stimulating effect on the activity of the heart, increases the strength and number of heart contractions, increases coronary blood flow and myocardial oxygen demand. Reduces the tone of blood vessels (mainly those of the brain, skin and kidneys). It has a peripheral venodilating effect, reduces pulmonary vascular resistance, and reduces pressure in the “lesser” circulation. Increases renal blood flow and has a moderate diuretic effect. Expands extrahepatic bile ducts. Inhibits platelet aggregation (suppresses platelet activating factor and PgE2 alpha), increases the resistance of red blood cells to deformation (improves the rheological properties of blood), reduces thrombus formation and normalizes microcirculation. It has a tocolytic effect, increases the acidity of gastric juice. When used in large doses, it has an enileptogenic effect.
Pharmacokinetics
After oral administration, it is quickly and completely absorbed, bioavailability is 90-100%. Food reduces the rate of absorption without affecting its magnitude (large volumes of liquid and proteins speed up the process). The higher the dose taken, the lower the absorption rate. The time to reach Cmax is 1-2 hours. Vd is in the range of 0.3-0.7 l/kg (30-70% of the “ideal” body weight), on average 0.45 l/kg. The connection with plasma proteins in adults is 60%, in patients with liver cirrhosis - 36%. Penetrates into breast milk (10% of the dose taken), through the placental barrier (the concentration in the fetal blood serum is slightly higher than in the maternal serum).
Aminophylline exhibits bronchodilating properties in concentrations of 10-20 mcg/ml. Concentrations above 20 mg/ml are toxic. The stimulating effect on the respiratory center is realized at a lower content of the drug in the blood of 5-10 mcg/ml. Metabolized at physiological pH values with the release of free theophylline, which is further metabolized in the liver with the participation of several cytochrome P450 isoenzymes. As a result, 1, 3-dimethyluric acid (45-55%) is formed, which has pharmacological activity, but is 1-5 times inferior to theophylline. Caffeine is an active metabolite and is formed in small quantities. In children over 3 years of age and in adults (unlike younger children), the phenomenon of caffeine accumulation is absent. T1/2 in children over 6 months – 3.7 hours; in adults – 8.7 hours; for “smokers” (20-40 cigarettes per day) - 4-5 hours (after quitting smoking, pharmacokinetics normalize after 3-4 months); in adults with chronic obstructive pulmonary disease, pulmonary heart disease and pulmonary heart failure - over 24 hours. Excreted by the kidneys.
Side effects
When taking Eufillin, in addition to the main pharmacological action, side effects may also occur. Most often, patients develop insomnia, dizziness, weakness, tachycardia, arrhythmia, dyspeptic syndrome, allergic reactions, hypoglycemia, hypotension, increased sweating, pain in the head, chest and abdomen.
Against the background of a drug overdose, muscle cramps, increased body temperature, polyuria, hematuria, albuminuria and even disturbances of consciousness may occur.
To eliminate adverse symptoms, it may be necessary to reduce the dosage or stop taking the drug.
If the rules of asepsis and antisepsis are violated during injections of Eufillin, infectious complications sometimes develop.