Pharmacological properties
Pharmacodynamics.
Tizanidine is a centrally acting skeletal muscle relaxant. the main point of application of its influence is the spinal cord. By stimulating presynaptic α2-adrenergic receptors, it inhibits the release of amino acids that stimulate n-methyl-d-aspartate receptors (nmda receptors). as a result, polysynaptic signal transmission at the level of interneuron connections in the spinal cord, which is responsible for excess muscle tone, is suppressed, and muscle tone decreases. Sirdalud is effective both for acute painful muscle spasms and for chronic spasticity of spinal and cerebral origin. it reduces resistance to passive movements and suppresses spasm and clonic convulsions and improves the strength of active muscle contractions. Pharmacokinetics
Absorption and distribution. Tizanidine is rapidly absorbed. Cmax in blood plasma is achieved approximately 1 hour after administration. The average absolute bioavailability is 34%. The mean steady state volume of distribution (Vss) after IV administration is 2.6 L/kg body weight. Binding to blood plasma proteins is 30%. The relatively low variation among patients in pharmacokinetic parameters (Cmax and AUC) facilitates reliable preliminary assessment of plasma levels after oral administration.
Metabolism/excretion. The drug undergoes rapid and extensive metabolism in the liver. Tizanidine is metabolized in vitro primarily by CYP 1A2. Metabolites are inactive. They are excreted primarily by the kidneys (70%). The elimination of total radioactivity (that is, the unchanged substance and metabolites) is two-phase, with a fast initial phase (T½ - 2.5 hours) and a slower elimination phase (T½ - 22 hours). Only a small amount of the substance unchanged (about 2.7%) is excreted by the kidneys. The average half-life of the substance unchanged is 2–4 hours.
Pharmacokinetics in certain groups of patients. In patients with renal failure (creatinine clearance 25 ml/min), the mean plasma Cmax is twice that of healthy volunteers, and the final half-life is increased to approximately 14 hours, resulting in an average 6-fold increase in AUC.
No studies have been conducted in patients with impaired liver function.
Tizanidine is metabolized by the CYP 1A2 isoenzyme in the liver. Patients with impaired liver function may exhibit higher plasma concentrations of the substance.
Sirdalud is contraindicated in persons with severe liver dysfunction.
Pharmacokinetic data in elderly patients are limited.
Gender does not affect the pharmacokinetic properties of tizanidine.
The influence of ethnicity and race on the pharmacokinetics of tizanidine has not been studied.
The influence of food. Concomitant food intake does not affect the pharmacokinetic profile of Sirdalud tablets. Although the Cmax value increases by a third, this is not clinically significant. No significant effect on absorption was noted.
Possibility of using the drug tizanidine (Sirdalud®) in patients with post-stroke spasticity
Post-stroke spasticity significantly limits the motor capabilities of patients, complicates patient care, and contributes to the formation of pain syndromes. The article presents a modern view of the pathophysiology of post-stroke spasticity and discusses approaches to treatment. The mechanisms of action and capabilities of the drug tizanidine (Sirdalud®) for the correction of increased muscle tone in patients who have suffered a stroke are considered. The effect of tizanidine is compared with the effect of other muscle relaxants.
Introduction
Spasticity is one of the most common motor deficits in stroke patients. During the first year after a stroke, spasticity is observed in 17–43% of patients [1, 2]. As a rule, spasticity is accompanied by paresis of the corresponding muscles, but there is no strict relationship between these symptoms.
Hemiparesis, a classic clinical example of spastic paresis after a stroke, is characterized by the formation of the Wernicke-Mann position: the shoulder is adducted to the body, the forearm is flexed, the hand is pronated and bent, the thigh and lower leg are extended, the foot is in a plantar flexion position. Due to the “lengthening” of the leg, the patient is forced to move it across the side when walking, describing a semicircle. These changes occur due to an increase in tone mainly in anti-gravity muscle groups. However, in some cases, other variants of the distribution of increased muscle tone are observed, for example, hyperpronation of the forearm with extension of the fingers, bizarre placement of the hand and fingers, increased tone in the leg flexor muscles, hypersupination of the forearm and wrist extensors [3].
Most often, spasticity is detected simultaneously in the upper and lower extremities (68%), but an isolated appearance of this symptom in the arm (15%) or leg (18%) is also possible [4].
Pathophysiology of spasticity
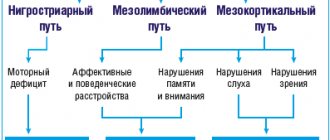
To date, the question of the pathogenesis of post-stroke spasticity remains open. It is known that this symptom occurs due to structural and functional changes in the central nervous system and at the level of the peripheral neuromotor apparatus. Thus, with spasticity, along with the activation of the cortical zones involved in the organization of movement and the extrapyramidal pathways of the brain stem, a structural and functional reorganization of the segmental structures of the spinal cord occurs. In addition, changes in the neuronal control of the peripheral part of the motor system activate processes of transformation of the protein composition of soft tissues, primarily skeletal muscles.
In response to cerebrovascular accidents and the development of a structural and functional defect of the brain, the functional activity of the cortex changes on both the affected and clinically intact sides. In addition, new neuronal connections are formed, and the area of representation of the affected part of the body in the motor and sensory cortex expands. In this case, an imbalance arises between intracortical excitation and inhibition towards the predominance of excitation [5–7]. In response to these changes, the extrapyramidal pathways of the brain stem are activated, which begin to generate spontaneous activity that is not associated with the motor activity of the cortex [6].
The main excitatory neurotransmitters of the nervous system involved in the regulation of muscle tone are glutamate and aspartate, the main inhibitory neurotransmitter is gamma-aminobutyric acid (GABA). A certain role in the implementation of muscle tone disorders is played by the serotonergic and noradrenergic systems of the brain stem, which can have both excitatory and inhibitory effects at the level of spinal neuronal networks [8].
A change in the functional interaction between different parts of the central nervous system leads to a reorganization of the segmental apparatus of the spinal cord. New contacts are formed between the neurons of the spinal cord and the descending pathways of the brain, which is accompanied by an increase in reflex excitability at the segmental level and is clinically realized by the formation of “dynamic” phenomena of spasticity in the form of clonus and synkinesis [6].
One of the methods for assessing the functional state of the segmental apparatus of the spinal cord is to study the parameters of the H-reflex (muscle response to irritation of sensory fibers of the peripheral nerve). It has been established that with spasticity, the zone of evocation of the H-reflex significantly expands [9].
Disruption of neuronal control of skeletal muscles leads to a transformation of their protein structure, primarily the myosin phenotype, as well as changes in the properties of the surrounding soft tissues (tendons and joint capsules). As a result, the proportion of “fast” but easily fatigued fibers in the muscle increases significantly, and tendon retraction leads to the formation of contractures [8, 10].
Spasticity of different muscle groups affects the patient's motor activity differently. For example, spasticity of the quadriceps femoris muscle in conditions of paresis can help maintain an upright posture by preserving the supporting function of the leg. At the same time, spasticity of the hand muscles always limits its functionality. In general, post-stroke spasticity has a negative impact on motor activity and is usually accompanied by a number of dynamic motor phenomena: involuntary movements, clonus or spasms, spastic synkinesis. In addition to the deterioration of motor functions, post-stroke spasticity can contribute to the development of post-stroke pain syndrome in the shoulder area, the prevalence of which varies from 16% in the early recovery period to 36% in the late recovery period [11].
The severity of clinical symptoms in post-stroke spasticity and its impact on the quality of life are assessed by the doctor, the patient, and caregivers. First of all, the degree of spasticity at rest is determined, for which the Ashworth scale is used, then the functional state of the limb during movement is assessed, as well as the presence of symptoms accompanying spasticity (pain, discomfort, pathological synkinesis). Taking into account all these factors, a treatment plan is developed.
Treatment
Patients with post-stroke spasticity are advised to undergo both non-drug and drug treatment.
Non-drug treatment includes physical exercise, positional therapy, the use of orthoses, massage, heat therapy, cryotherapy, electrical myostimulation, and biofeedback methods [3, 12]. Since these activities, as a rule, have a short-term effect, they should be carried out continuously, not limited to one or two courses per year. In this regard, it is necessary to train patients and their caregivers in techniques that can be used at home.
The optimal medical method for correcting local spasticity is considered to be local injections of botulinum toxin, which consists of neurotoxin type A and some other proteins. Botulinum toxin blocks the release of acetylcholine into the synaptic cleft. The most significant results of botulinum therapy include improved walking and increased self-care capabilities. The effect develops after four to seven days and lasts in most cases for 12–16 weeks [13]. However, the maximum effectiveness of the drug is achieved when it is administered under electromyography control, which is not always available. In addition, injections of the drug are not recommended for patients taking therapeutic doses of anticoagulants [13]. In general, despite the development of care for patients who have suffered a stroke, today the possibilities of botulinum therapy are limited.
Another method of local exposure is the use of alcoholic and phenolic neurolysis of the motor branches of nerves, which leads to their irreversible destruction and fibrosis of surrounding tissues. The procedure is carried out under the control of electromyography and/or ultrasound [13]. The disadvantage of this method is side effects such as dysesthesia, weakness and local swelling at the injection site [1]. Currently, neurolysis is rarely used (mainly in bedridden patients), usually in combination with botulinum therapy. The effect lasts from two to 36 months [13].
Of the first-line oral medications used for post-stroke spasticity, international recommendations indicate centrally acting muscle relaxants: tizanidine and baclofen. It is also possible to use tolperisone, diazepam, gabapentin [13]. In the treatment of cerebral spasticity, tizanidine is most often used; for spinal spasticity, tizanidine and baclofen are used [14].
Tizanidine in the treatment of post-stroke spasticity
Tizanidine (Sirdalud®) is a central agonist of imidazoline and alpha-2 adrenergic receptors at both the spinal and supraspinal levels [13, 15]. Tizanidine enhances presynaptic inhibition of motor neurons by reducing the release of excitatory amino acids (aspartate and glutamate) from spinal interneurons, as well as by inhibiting the activity of facilitatory cerebrospinal tracts. In addition, tizanidine prevents the release of substance P by thin sensory fibers, helps to reduce the functional activity of the locus coeruleus region in the brain stem and reduce the excitability of the segmental apparatus of the spinal cord [16].
Controlled clinical studies have shown the effectiveness of tizanidine in post-stroke spasticity. Thus, one study showed that tizanidine at a dose of 8 mg/day reduces the level of H-reflex facilitation in all patients (n = 14) with post-stroke spasticity. This does not happen when using a placebo. Decreased facilitation was observed on both the affected and clinically intact sides. At the same time, when taking tizanidine, there is no change in the Hmax/Mmax indicator and the threshold for evoking the H-reflex, which probably indicates the absence of the drug’s effect on the excitability of spinal motor neurons. There was a significant decrease in the degree of spasticity from 2.9 to 1.9 points on the Ashworth scale [17].
Several studies have shown a dose-dependent antispastic effect of tizanidine. In a group of patients with multiple sclerosis (n = 17) and a degree of spasticity in the lower extremities of two to three points on the Ashworth scale, a relationship was shown between the concentration of the drug in the blood and its antispastic effect. At the same time, no connection was found between certain values of the drug concentration and the antispastic effect in the examined group, which indicates the need for individual dose selection. It was noted that the effect of 2 mg tizanidine was no different from that of placebo. Thus, the degree of spasticity two hours after taking 2 mg of the drug decreased by 29% (when taking placebo - by 28%), when taking 8 mg of tizanidine - by 38%. The antispastic effect was not accompanied by a decrease in muscle strength. In this study, data from a clinical assessment of the degree of spasticity were confirmed by the results of a quantitative study of muscle tone using an electrogoniometer (the device showed a high degree of sensitivity and reliability) [8].
The dependence of the antispastic effect on the dose of tizanidine in patients with post-stroke spasticity was studied in a multicenter study that included 47 patients, the degree of spasticity was two to three points on the Ashworth scale, the time since stroke was six months [16]. The dose of the drug was titrated to 36 mg (reaching the maximum dosage in 21% of patients). As a result of taking tizanidine over 16 weeks, the degree of spasticity decreased without changing muscle strength, the intensity of pain syndrome and, as a result, the quality of life of patients improved [16].
Thus, studies have confirmed the dose-dependent clinical effect of the drug; the treatment of spasticity requires the use of higher doses of tizanidine than the treatment of pain syndromes. The lack of effect in some cases may be due to an insufficient dose of the drug [8, 18].
An analysis of the results of several studies examining the antispastic effect of tizanidine compared with placebo and other oral muscle relaxants showed a reduction in spasticity with tizanidine of 21-37%, while for placebo this figure ranged from 4 to 9%. At the same time, muscle tone decreased in 60–80% of patients in the tizanidine group, in 60–65% in the baclofen group, and in 60–83% in the diazepam group. Tolerability of the drug was assessed as very good in 44–100% of patients in the tizanidine group, 38–90% in the baclofen group and 20–54% in the diazepam group. However, in the tizanidine group, patients did not experience an increase in muscle weakness, unlike patients taking baclofen. All drugs caused drowsiness [19]. Based on a combination of efficacy and tolerability indicators, tizanidine demonstrated significant advantages in the treatment of post-stroke spasticity compared to other oral muscle relaxants [16, 19, 20].
Studies comparing the treatment effects of tizanidine and botulinum toxin for post-stroke spasticity in the upper extremity have shown that both drugs are effective. At the same time, to achieve optimal results, it is necessary to accurately follow the dosage and determine the injection sites of botulinum toxin [18]. Another study examined the combined use of botulinum toxin with oral antispasticity drugs in a group of children with cerebral palsy. The combination of botulinum toxin with tizanidine has been found to be more effective than the combination of botulinum toxin and baclofen [21].
When taken orally, tizanidine is rapidly absorbed, with maximum plasma concentrations achieved within one to two hours. The optimal therapeutic effect, as a rule, develops when the drug is prescribed in a daily dose of 12–24 mg, divided into three doses; the effective dose range is 2–36 mg [22]. The maximum daily dose is 36 mg. Increasing and decreasing the dosage should be done gradually. Tizanidine is metabolized in the liver mainly by the CYP1A2 isoenzyme. It should be remembered: drugs such as ciprofloxacin and fluvoxamine inhibit this enzyme, as a result of which the hypotensive effect of tizanidine can be significantly enhanced. Particular caution should be exercised when combining the drug with antihypertensive drugs. Abrupt discontinuation of tizanidine may lead to withdrawal syndrome, especially with long-term treatment in conjunction with antihypertensive drugs.
The most common side effects observed while taking tizanidine are drowsiness, dizziness, and decreased blood pressure. General weakness, dry mouth, sleep disturbances, and hallucinations are also possible [8, 17]. It should be noted that all side effects are dose-related and can be minimized with proper dose titration. If they appear, the dosage should be reduced to the previous level for several days, and then the dose increase should be resumed [8]. As a rule, with poor tolerability, side effects appear even at minimal dosages of the drug [8, 22].
Currently, tizanidine is also available in a 6 mg dose in the form of modified-release capsules (Sirdalud® MP). For most patients, the optimal dose is 12 mg/day, in rare cases – 24 mg/day [22]. Treatment begins with one capsule per day, if necessary, the dose is increased every seven days. It should be noted that Sirdalud® MR has shown high effectiveness against central sensitization in the development of post-stroke pain syndrome in the shoulder area [11].
Other muscle relaxants
Another first-line drug for the treatment of post-stroke spasticity is baclofen, an agonist of B-type GABA receptors located in the area of the endings of the primary sensory afferents of the spinal cord. By enhancing the polarization of interneuron membranes, baclofen prevents the flow of calcium into presynaptic terminals and the release of endogenous transmitters, resulting in inhibition of mono- and polysynaptic reflexes of the spinal cord [13].
The maximum dosage is 120 mg/day, changing the dose up or down should be done once a week and by no more than 15 mg. Side effects of baclofen are also dose-dependent: general weakness and drowsiness are most often noted. Experimental studies in animals have shown that GABAergic drugs may reduce brain plasticity in the early recovery period after stroke, and therefore it is not advisable to prescribe them at this time. The drug is also not recommended for elderly patients due to the development of severe drowsiness [13].
Attention should be paid to the possibility of intrathecal administration of baclofen using a pump. This is a highly effective treatment method for severe spasticity, including in patients who have suffered a stroke, which significantly reduces the incidence of side effects. However, this method is used relatively rarely, which is associated with the high cost of equipment [13].
In our country, tolperisone is widely used - a centrally acting muscle relaxant, similar in structure to lidocaine. The drug stabilizes nerve cell membranes. The use of tolperisone has shown high efficacy and safety in reducing muscle tone in spasticity in several controlled studies [23, 24].
In Russia, a comparative study was conducted on the effectiveness of various muscle relaxants in patients with post-stroke spasticity, the results of which showed a decrease in the degree of spasticity and an improvement in everyday adaptation in patients taking tolperisone and tizanidine. In this case, tolperisone was prescribed at a dose of up to 900 mg/day. During the study, no significant side effects were identified when taking tolperisone [23]. However, there have been no large international studies of this drug in post-stroke spasticity. It is advisable to start treatment with tolperisone with a daily dose of 300 mg, which, if necessary, can be increased to 900 mg.
Other oral medications used to reduce muscle tone in spasticity are benzodiazepines, gabapentin and dantrolene (not registered in Russia). However, no data have been obtained on their effectiveness in post-stroke spasticity [13].
The literature discusses the possibility of combining various muscle relaxants in order to achieve maximum effect. To date, there is no evidence base for recommending any combinations of drugs; however, the likelihood of additive side effects increases, and therefore attempts to combine oral muscle relaxants seem irrational [13].
Conclusion
Regardless of the severity of post-stroke spasticity, it should be remembered that spasticity increases significantly in the presence of pain syndromes, bedsores, constipation, and urinary tract infections. Treatment of these conditions, as well as providing a comfortable environment, are necessary conditions for correcting spasticity and improving the patient’s quality of life. Pharmacological treatment should be individualized and carried out over a long period of time. The dose of the selected drug should be increased gradually until a clinical effect is achieved. In the absence of significant effect from oral medications, it is necessary to consider a combination of pharmacotherapy and surgical methods for the treatment of post-stroke spasticity.
Application
Sirdalud has a narrow therapeutic range and high interpatient variability in tizanidine plasma concentrations. therefore, it is important to use it in optimal doses according to the patient's needs. Treatment should be started with a low dose of 2 mg, which makes the risk of unwanted effects from taking the drug minimal. if necessary, the dose of the drug can be gradually increased while observing all the required precautions.
Adults
Relief of painful muscle spasms. Apply 2–4 mg 3 times a day. In severe cases, an additional dose of 2 or 4 mg may be taken at bedtime.
Spasticity in neurological disorders. The dose should be selected individually for each patient.
The initial daily dose should not exceed 6 mg, divided into 3 doses. It can be increased gradually to 2–4 mg 2 times at intervals of 3–7 days. Typically, the optimal therapeutic effect is achieved with a daily dose of 12–24 mg, divided into 3 or 4 doses. The total daily dose should not exceed 36 mg.
Special patient populations
Use in children and adolescents. Experience with the use of Sirdalud in children and adolescents is limited, so it is not recommended for use in this category of patients.
Use in elderly people. Experience with the use of the drug in elderly patients is limited, so caution should be exercised when using the drug Sirdalud in this category of patients. It is recommended to start treatment with a minimum dose and gradually, with caution, increase it in small steps until the optimal ratio of individual tolerability and therapeutic effectiveness of the drug is achieved.
Use in patients with impaired renal function. For patients with impaired renal function (creatinine clearance 25 ml/min), the recommended initial single daily therapeutic dose is 2 mg. The dose is increased gradually and with caution, in “small steps,” until the optimal balance of individual tolerability and therapeutic effectiveness of the drug is achieved. In order to increase therapeutic effectiveness, you should first increase the single dose before moving on to more frequent dosing of the drug during the day.
Use in patients with impaired liver function. Treatment of persons with severe liver dysfunction is contraindicated. Sirdalud is largely metabolized in the liver. Sirdalud should be used with caution when treating patients with moderately severe liver dysfunction. Treatment must be started with a minimum dose; possible dose increases must be carried out with caution and taking into account the patient’s individual tolerance to the drug Sirdalud.
Interruption of treatment. If it is necessary to interrupt treatment, the dose should be reduced slowly and gradually. This is especially true for patients who have used the drug in high doses for a long time. This reduces the risk of developing a rebound increase in blood pressure and tachycardia.
Comparison of ease of use of Tizanidin-teva and Sirdalud
This includes dose selection taking into account various conditions and frequency of doses. At the same time, it is important not to forget about the release form of the drug; it is also important to take it into account when making an assessment.
The ease of use of Tizanidin-teva is approximately the same as Sirdalud. However, they are not convenient enough to use.
The drug ratings were compiled by experienced pharmacists who studied international research. The report is generated automatically.
Last update date: 2020-12-04 13:48:04
Side effects
Adverse reactions - such as drowsiness, fatigue, dizziness, dry mouth, decreased blood pressure, nausea, gastrointestinal disorders and increased levels of transaminases in the blood plasma - are usually mild and transient in patients using the drug in low doses, recommended for relief painful muscle spasm.
When taken in higher doses than recommended for spasticity, the above adverse reactions occur more frequently and are more severe, but they are rarely severe enough to warrant discontinuation of treatment. The following adverse reactions may also occur: hypotension, bradycardia, muscle weakness, sleep disturbance, hallucinations and hepatitis.
The appearance of such symptoms has been reported after sudden discontinuation of tizanidine, especially after long-term treatment and/or use in high daily doses and/or concomitant therapy with antihypertensive drugs. Under such circumstances, patients may experience hypertension and tachycardia. In some cases, such rebound hypertension can cause a stroke. Therefore, treatment with tizanidine should not be stopped suddenly, but only by gradually reducing the dose.
To assess the incidence of various adverse reactions, the following classification was used: very often (≥1/10), often (≥1/100, 1/10), uncommon (≥1/1000, 1/100), rarely (≥1/10 000, 1/1000), very rarely (1/10,000), including individual messages.
Mental disorders: often - insomnia, sleep disturbance.
From the side of the central nervous system: very often - drowsiness, dizziness; frequency unknown - confusion, vertigo.
From the heart: infrequently - bradycardia
From the vascular system: often - arterial hypotension; slight decrease in blood pressure.
From the digestive tract: very often - dry mouth, gastrointestinal disorders; often - nausea.
Hepatobiliary disorders: often - increased levels of transaminases in the blood plasma.
From the musculoskeletal system: very often - muscle weakness.
General disorders: very often - increased fatigue.
Post-marketing studies
Additional adverse reactions to the drug were reported in post-marketing studies.
These adverse reactions have been reported from an unknown number of patients, so their frequency cannot be reliably estimated.
From the immune system: hypersensitivity reactions (including anaphylaxis, swelling of the throat, shortness of breath and urticaria).
Mental disorders: hallucinations, confusion.
From the side of the central nervous system: vertigo.
From the cardiovascular system: syncope.
From the side of the organ of vision: blurred vision.
Hepatobiliary disorders: hepatitis, liver failure.
From the skin and subcutaneous tissue: rash, erythema, itching, dermatitis.
General disorders: asthenia, withdrawal syndrome.
special instructions
Concomitant use of cyp 1a2 inhibitors with tizanidine is not recommended.
After abrupt discontinuation of the drug or rapid dose reduction, patients may experience hypertension and tachycardia. In some cases, such rebound hypertension can cause a stroke. Treatment with tizanidine should not be stopped suddenly, but only by gradually reducing the dose.
For patients with renal failure (creatinine clearance 25 ml/min), the initial dose is 2 mg 1 time per day. The dose should be increased sequentially, in small "steps", taking into account effectiveness and tolerability. To achieve a more pronounced effect, it is recommended to first increase the dose prescribed once a day, and then increase the frequency of administration.
Liver failure has been reported in association with tizanidine, but this has been reported rarely in patients receiving daily doses up to 12 mg. In this regard, it is recommended to monitor liver function once a month during the first 4 months of therapy in patients using tizanidine at a dose of ≥12 mg and in patients with clinical symptoms suggestive of liver failure (for example, nausea, loss of appetite, or fatigue of unknown origin). etiology). Sirdalud should be discontinued if plasma ALT or AST levels exceed the upper limit of normal by ≥3 times the upper limit of normal for a prolonged period.
Close monitoring of patients is recommended for 1 or 2 days after taking the first dose of tizanidine. In case of development of anaphylaxis or swelling of the throat with anaphylactic shock or shortness of breath, the use of the drug Sirdalud should be stopped immediately and the patient prescribed the necessary treatment.
Arterial hypotension may occur with the use of tizanidine, as well as as a result of drug interactions with CYP 1A2 inhibitors and/or antihypertensive drugs. Severe forms of hypotension, such as loss of consciousness and circulatory collapse, have been reported.
Caution should be exercised when using this drug with drugs that prolong the QT interval (eg, cisapride, amitriptyline, azithromycin).
Caution is necessary for patients with coronary artery disease and/or heart failure. ECG monitoring should be carried out at regular intervals when starting the use of Sirdalud in these patients.
Before using this drug, patients with myasthenia gravis should carefully evaluate the risk/benefit ratio.
Experience in children and adolescents is limited, therefore the use of Sirdalud is not recommended in this category of patients.
Caution should be exercised when using this drug in elderly people.
Sirdalud tablets contain lactose. For patients with rare hereditary diseases - galactose intolerance, severe lactase deficiency or glucose-galactose malabsorption syndrome - Sirdalud tablets are not recommended.
Use during pregnancy and lactation
Women of childbearing age. Women of childbearing age who are sexually active should undergo a pregnancy test before starting treatment with Sirdalud. Women of childbearing potential should be advised that animal studies indicate that Sirdalud may have adverse effects on the fetus. Women of childbearing potential who are sexually active should use effective contraceptive methods (methods that allow pregnancy in less than 1% of cases) throughout the entire period of treatment with Sirdalud and for 1 day after stopping treatment with the drug.
Pregnancy. Data on the use of Sirdalud in pregnant women are limited, so it should not be prescribed during pregnancy, unless the potential benefit to the mother outweighs the possible risk to the fetus.
Lactation. No teratogenic effects were observed when tizanidine was used in rats and rabbits. Animal experiments have shown that tizanidine passes into breast milk in small quantities. Therefore, women who are breastfeeding should not prescribe the drug.
Fertility. No impairment of fertility was observed in male rats receiving the drug at a dose of 10 mg/kg/day and in female rats receiving the drug at a dose of 3 mg/kg/day. A decrease in fertility was detected in male rats receiving the drug at a dose of 30 mg/kg/day, and in female rats receiving the drug at a dose of 10 mg/kg/day. When using the drug in these doses, sedation, weight loss and ataxia were also noted.
Children. Experience with the drug in pediatrics is limited. It is not recommended to prescribe Sirdalud to children.
The ability to influence reaction speed when driving vehicles or working with other mechanisms. Tizanidine may cause drowsiness, dizziness and/or hypotension, thereby impairing the patient's ability to drive or operate machinery. The risks increase with simultaneous use of alcohol.
Therefore, you should refrain from activities that require a high concentration of attention and quick reactions, such as driving vehicles or working with machines and mechanisms.
Comparison of habituation in Tizanidin-teva and Sirdalud
Like safety, addiction also involves many factors that must be considered when evaluating a drug.
Thus, the totality of the values of such parameters as “o syndrome” in Tizanidin-teva is quite similar to the similar values in Sirdalud. Withdrawal syndrome is a pathological condition that occurs after the cessation of intake of addictive or dependent substances into the body. And resistance is understood as initial immunity to a drug; in this it differs from addiction, when immunity to a drug develops over a certain period of time. The presence of resistance can only be stated if an attempt has been made to increase the dose of the drug to the maximum possible. At the same time, in Tizanidin-teva the meaning of the “syndrome” is quite small, however, the same as in Sirdalud.
Interactions
Concomitant use of known cyp 1a2 inhibitors may increase plasma levels of tizanidine. An increase in tizanidine plasma levels may lead to symptoms of overdose, such as prolongation of the qt interval.
Concomitant use of known CYP1A2 inducers may reduce plasma levels of tizanidine. A decrease in the plasma level of tizanidine may lead to a decrease in the therapeutic effect of Sirdalud.
Concomitant use of strong CYP1A2 inhibitors such as fluvoxamine or ciprofloxacin with tizanidine is contraindicated. Coadministration of tizanidine with fluvoxamine increased tizanidine AUC by 33-fold, while coadministration of tizanidine with ciprofloxacin increased tizanidine AUC by 10-fold. This can lead to a clinically significant and long-lasting decrease in blood pressure, accompanied by drowsiness, dizziness, and decreased psychomotor performance.
Concomitant use of tizanidine with other CYP1A2 inhibitors such as antiarrhythmic drugs (amiodarone, mexiletine, propafenone), cimetidine, some fluoroquinolones (enoxacin, pefloxacin, norfloxacin), rofecoxib, oral contraceptives and ticlopidine is not recommended.
Elevated plasma levels of tizanidine may cause overdose symptoms, including prolongation of the QT interval.
Concomitant use of Sirdalud with antihypertensive drugs, including diuretics, can sometimes cause arterial hypotension and bradycardia. Some patients receiving concomitant treatment with antihypertensive drugs experienced rebound hypertension and rebound tachycardia when tizanidine was abruptly discontinued. In some cases, rebound hypertension can cause a stroke.
The combined use of Sirdalud with rifampicin can lead to a 50% decrease in tizanidine concentrations. Therefore, the therapeutic effect may be reduced when rifampicin is used during therapy with Sirdalud, which may be clinically significant in some patients. Prolonged concomitant use should be avoided and, if necessary, the dose should be adjusted very carefully.
The use of the drug Sirdalud leads to a 30% reduction in the systemic effect of tizanidine in smokers (more than 10 cigarettes per day). Long-term use of the drug in patients who smoke a lot requires the use of the drug in higher doses.
The simultaneous use of Sirdalud and other centrally acting drugs (for example, sedatives and hypnotics (benzodiazepine or baclofen), some antihistamines and analgesics, psychotropic drugs, narcotics) may increase the severity of the effects of each drug and enhance the hypnotic effect of Sirdalud. This applies, in particular, to the simultaneous use of alcohol, which can unexpectedly change or enhance the effect of Sirdalud and increase the risk of adverse reactions, so you should refrain from drinking alcohol.
The use of Sirdalud concomitantly with α2-adrenergic agonists (eg clonidine) should be avoided due to their potential additive hypotensive effect.
Comparison of the effectiveness of Tizanidin-teva and Sirdalud
Sirdalud is more effective than Tizanidine-teva - this means that the ability of the drug substance to provide the maximum possible effect is different.
For example, if the therapeutic effect of Sirdalud is more pronounced, then it is impossible to achieve this effect with Tizanidin-teva even in large doses.
Also, the speed of therapy is an indicator of the speed of the therapeutic action; Sirdalud and Tizanidin-teva are also different, as is bioavailability - the amount of the drug reaching the site of its action in the body. The higher the bioavailability, the less it will be lost during absorption and use by the body.
Overdose
There have been very few reports of overdose with sirdalud. All patients with isolated cases of overdose of this drug, including 1 patient who took 400 mg of sirdalud, recovered without complications.
Symptoms: nausea, vomiting, hypotension, bradycardia, prolongation of the QT interval, dizziness, miosis, respiratory distress, coma, anxiety, drowsiness.
Treatment. To remove the drug from the body, repeated use of activated carbon in high doses is recommended. Forced diuresis may speed up the elimination of the drug. In the future, symptomatic treatment should be carried out.
Note!
Description of the drug Sirdalud table. 4mg No. 30 on this page is a simplified author’s version of the apteka911 website, created on the basis of the instructions for use.
Before purchasing or using the drug, you should consult your doctor and read the manufacturer's original instructions (attached to each package of the drug). Information about the drug is provided for informational purposes only and should not be used as a guide to self-medication. Only a doctor can decide to prescribe the drug, as well as determine the dose and methods of its use.