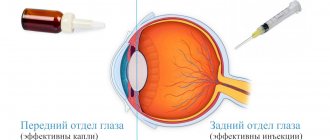
Conservative treatment methods are an integral component of the treatment program for eye diseases. One type of such treatment is intravitreal injections - a method in which medications are injected directly into the eye cavity through injections. This type of treatment is indicated for patients with pathologies of the posterior part of the eye (retina, optic nerve, vitreous) due to the fact that drops and tablets for these diseases are much less effective.
A drug introduced into the eye cavity requires the use of a smaller amount and a lower concentration of the drug substance, while it becomes possible to preserve the medicinal properties of the drug for a long time (from two weeks to two months) directly at the site of the lesion, in this case - inside the eye.
Our specialized ophthalmology center is one of the most experienced medical institutions providing services to patients with retinal pathologies. Every month, hundreds of microsurgical operations are performed within the walls of our clinic; they are performed by highly qualified specialists with many years of clinical experience.
Pharmacodynamics and pharmacokinetics
It has been established that Vitreous injections are prescribed when resorption of postoperative scars or burn scars is required, as well as to reduce pain caused by various neuralgic diseases, for example, radiculitis . This substance, obtained from the eyeball of an animal, contains various amino acids necessary for the formation of muscle tissue. In addition, the substance contains hyaluronic acid , which ensures the normal functioning of heart valves and joints.
Despite the fact that this drug is a natural product of animal origin, there are a number of contraindications for its use, so treatment is carried out only after consultation with a doctor.
How does the procedure work?
In terms of their effectiveness, inravitreal injections are comparable to some types of vitreoretinal operations. The procedure requires sterile conditions and therefore must be performed in a specially equipped suitable room, such as an operating room. To perform injections into the eyeball, local anesthesia is used with eye drops, in order to avoid complications, the patient is prescribed antiseptics.
During the administration of the drug into the eye cavity, an anesthesiologist is present in the operating room, who monitors the patient’s vital functions and ensures the proper level of pain relief for the patient. The requirements for asepsis and antisepsis during intravitreal injection manipulation are the same as when performing vitreoretinal surgery and must comply with international standards for intraocular surgical interventions.
At the initial stage, the patient's eyeball is fixed using an eyelid expander, after which the doctor marks the area for future intraocular injection in the lower outer quadrant of the eyeball, 3.5 mm from the limbus. This allows the doctor to avoid future trauma to intraocular structures (lens, ciliary body, retina) and reduce the likelihood of intraocular complications. The drug is injected directly into the vitreous body of the eye through the outer membranes of the eye; for this purpose, special needles are used to prevent the drug from leaking out when the needle is removed. Due to the fact that the manipulation is carried out using an operating microscope, the doctor controls the process of drug administration and the depth of needle insertion. All of the above points make the procedure safe and minimize the risks of complications. Equally important is strict adherence to the recommendations of the attending physician in the postoperative period and regular postoperative monitoring.
Our clinic is equipped with the most modern equipment; we provide European-level services without queues and at affordable prices. When performing medical procedures, our specialists use the best equipment available today, including disposable instruments.
Indications for use
The drug Vitreous is prescribed:
- for the purpose of softening or resolving scar tissue formed as a result of burns , surgical interventions, and so on;
- for rapid formation of callus during fractures ;
- when you need to reduce pain due to radiculitis, neuralgia ;
- and also to improve joint mobility, for example, with contractures and other cases.
TielVel® ()
The dosage form for intravenous use should not be administered intramuscularly.
Dosing recommendations for imipenem + [cilastatin] indicate the amount of imipenem to be administered.
Calculation of the total daily dose of imipenem + [cilastatin] should be based on the severity of the infection and divided into several applications in equal doses, taking into account the degree of susceptibility of one or more pathogenic microorganisms and renal function.
Dosage schedule for adult patients:
Dosage selection of imipenem + [cilastatin] for the treatment of adult patients should be based on suspected or confirmed organism susceptibility to imipenem, as shown in Table 1. Dosing recommendations for imipenem + [cilastatin] are based on the amount of imipenem administered; an equivalent amount of cilastatin is also present in the solution. These doses should be used in patients with creatinine clearance > 90 ml/min. A dose reduction is necessary for patients with creatinine clearance <90 mL/min, as shown in Table 2 (see Dosage Schedule for Adults with Renal Impairment).
It is recommended that the total daily dose of the drug does not exceed 4 g.
A dose of imipenem + [cilastatin] for intravenous infusion equal to 500 mg should be administered intravenously over 20-30 minutes. A dose of 1000 mg should be administered intravenously over 40-60 minutes. Patients who experience nausea during the infusion should slow down the rate of drug administration.
Table 1. Imipenem + [cilastatin] dosing regimen for intravenous infusion in adult patients with creatinine clearance > 90 ml/min
| Suspected or confirmed sensitivity of the microorganism | Dose of imipenem + [cilastatin] for intravenous infusion |
| If the infection is suspected or confirmed to be caused by susceptible strains of bacteria | 500 mg every 6 hours |
| or | |
| 1000 mg every 8 hours | |
| If the infection is suspected or confirmed to be caused by intermediate-resistant strains of bacteria | 1000 mg every 6 hours |
Dosage schedule for adult patients with renal impairment:
In patients with creatinine clearance <90 mL/min, a reduction in the dose of imipenem + [cilastatin] for intravenous infusion is necessary, as shown in Table 2. Serum creatinine should indicate a stable state of renal function. To calculate creatinine clearance, use the Cockcroft-Gault method described below:
for men:
for women:
0.85 x (creatinine clearance value calculated for men)
Table 2. Dosing regimen of imipenem + [cilastatin] for intravenous infusion in adult patients with impaired renal function
| Creatinine clearance (ml/min) | ||||
| >90 | From > 60 to < 90 | >30 to <60 | From > 15 to < 30 | |
| Dose of imipenem + [cilastatin] for intravenous infusion if the infection is suspected or confirmed to be caused by susceptible strains of bacteria *° | 500 mg every 6 hours | 400 mg every 6 hours | 300 mg every 6 hours | 200 mg every 6 hours |
| or | ||||
| 1000 mg every 8 hours | 500 mg every 6 hours | 500 mg every 8 hours | 500 mg every 12 hours | |
| Dose of imipenem + [cilastatin] for intravenous infusion if the infection is suspected or confirmed to be caused by intermediate-resistant bacterial strains (see Pharmacodynamics)*0 | 1000 mg every 6 hours | 750 mg every 8 hours | 500 mg every 6 hours | 500 mg every 12 hours |
*Each dose of imipenem + [cilastatin] intravenous infusion less than or equal to 500 mg should be administered intravenously over 20 to 30 minutes.
° Each dose over 500 mg should be administered intravenously over 40-60 minutes.
Patients who experience nausea during the infusion should slow down the rate of drug administration.
Patients with creatinine clearance greater than or equal to 15 ml/min but less than 30 ml/min may have an increased risk of developing seizures (see section "Special Instructions"). Patients with a creatinine clearance of less than 15 mL/min should not receive imipenem + [cilastatin] intravenous infusion unless hemodialysis will be performed no later than 48 hours after the imipenem + [cilastatin] infusion. There is not enough information to recommend the use of imipenem + [cilastatin] intravenous infusion in patients receiving peritoneal dialysis.
Dosage schedule for patients on hemodialysis:
When treating patients with creatinine clearance less than 15 ml/min who are on hemodialysis, the dosage recommendations given in Table 2 (see “Dosing regimen for adult patients with impaired renal function”) should be used for patients with creatinine clearance less than 30 ml/ min, but greater than or equal to 15 ml/min.
Both imipenem and cilastatin are eliminated from the circulatory system during hemodialysis. Therefore, imipenem + [cilastatin] for intravenous infusion should be administered to patients after hemodialysis and at certain intervals after the end of the hemodialysis session. Patients undergoing hemodialysis, especially those with diseases of the central nervous system, should be closely monitored; Prescribing the drug to patients undergoing hemodialysis is recommended only in cases where the benefit of treatment outweighs the potential risk of developing seizures (see section "Special Instructions").
Elderly patients
For elderly patients with normal renal function, no dose adjustment is required.
Liver dysfunction
For patients with impaired liver function, no dose adjustment is required.
Dosage schedule for children from 3 months of age
For children, the following dosage regimen is recommended:
— Children weighing > 40 kg should receive the same doses as adult patients.
— Children over 3 months old and weighing less than 40 kg should receive the drug at a dose of 15 mg/kg at 6-hour intervals. The maximum daily dose should not exceed 2 g.
Imipenem + [cilastatin] is not recommended for the treatment of meningitis. If meningitis is suspected, appropriate antibiotics should be prescribed.
Preparation of imipenem + [cilastatin] solution for intravenous infusion
Imipenem + [cilastatin] for intravenous infusion is supplied in a vial containing one dose of dry powder, which must be reconstituted and further diluted using aseptic technique to obtain a solution for infusion.
To prepare a solution for infusion, the contents of the vial must be reconstituted by adding 10 ml of a suitable solvent to the vial.
The list of suitable solvents is as follows:
— 0.9% sodium chloride solution for injection;
— 5% dextrose solution for injection;
— 5% dextrose solution for injection + 0.9% sodium chloride solution for injection;
— 5% dextrose solution for injection + 0.45% sodium chloride solution for injection;
— 5% dextrose solution for injection + 0.225% sodium chloride solution for injection.
1) Remove 20 ml of diluent (2 x 10 ml) from the appropriate infusion container.
2) Reconstitute the contents of the bottle with 10 ml of solvent.
The reconstituted suspension cannot be used for intravenous administration.
3) The bottle containing the reconstituted suspension must be thoroughly shaken and the resulting suspension must be transferred to the remaining 30 ml of solvent in the infusion container.
4) To ensure quantitative transfer of the contents of the bottle, it is necessary to add 10 ml of the previously extracted solvent to the bottle and shake thoroughly. Repeat transferring the resulting suspension into the infusion container.
5) The final infusion solution must be shaken until a clear solution is obtained.
The color of imipenem + [cilastatin] solutions can vary from colorless to yellow (color changes within these limits do not affect the activity of the drug).
For patients with renal impairment, dose reduction will depend on creatinine clearance, as shown in Table 3.
1) Prepare 50 ml or 100 ml of solution for infusion as indicated above.
2) Select the volume of final infusion solution (ml) required for the appropriate dose of imipenem + [cilastatin] as shown in Table 3.
Drugs for parenteral use should be visually inspected for visible particles and discoloration whenever the solution and container allow this. Discontinue infusion if visible particles or discoloration are detected.
Table 3. Preparation of imipenem + [cilastatin] for intravenous infusion. Doses.
| Clearance creatinine, ml/min | Drug dose (imipenem/cilastatin), mg | Volume removed from the prepared solution, ml | Volume of final infusion solution for the required dose, ml |
| >90 | 500/5001 | — | 100 |
| From > 60 to < 90 | 400/4001 | 20 | 80 |
| From > 30 to < 60 | zoo/zoo1 | 40 | 60 |
| From > 15 to < 30 | 200/2002 | 10 | 50 |
1 - you should use 2 bottles of the drug imipenem + [cilastatin] with a dosage of 250 mg + 250 mg;
2 - you should use 1 bottle of the drug imipenem + [cilastatin] with a dosage of 250 mg + 250 mg.
Storage of imipenem + [cilastatin] solution for intravenous infusion after recovery
Imipenem + [cilastatin] powder for solution for infusion is supplied in single-dose vials and is reconstituted by adding a suitable diluent to the vial (see “Preparation of imipenem + [cilastatin] solution for intravenous infusion”). The prepared solution for infusion remains active for 4 hours when stored at room temperature or for 24 hours when stored in the refrigerator (5 ° C). Imipenem + [cilastatin] solution for intravenous infusion should not be frozen.
Table 4 presents data on the timing of use of imipenem + [cilastatin] infusion solution prepared from a number of infusion solvents and stored at room temperature or in the refrigerator.
Table 4.
| Solvent | Drug stability period | |
| Room temperature (25 °C) | Refrigerator (4 °C) | |
| 0.9% sodium chloride solution for injection | 4 hours | 24 hours |
| 5% dextrose solution for injection | 4 hours | 24 hours |
| 5% dextrose solution for injection + 0.9% sodium chloride solution for injection | 4 hours | 24 hours |
| 5% dextrose solution for injection + 0.45% sodium chloride solution for injection | 4 hours | 24 hours |
| 5% dextrose solution for injection + 0.225% sodium chloride solution for injection | 4 hours | 24 hours |
Contraindications for use
The use of the drug is not recommended for:
- infectious diseases;
- acute inflammatory processes;
- cachexia;
- jade;
- nephrosclerosis;
- liver cirrhosis;
- congestive heart failure;
- malignant tumors.
Vitreous body 2ml No. 10 ampoules
Vitreous body 2ml No. 10 ampoules
Trade name Vitreous humor International nonproprietary name No Dosage form Solution for injection 2 ml Composition per ampoule active substance: vitreous humor – 2 ml Description Transparent slightly yellowish liquid. Light opalescence and the presence of fine crystalline and tissue suspensions are allowed. Pharmacotherapeutic group Other therapeutic products. Other medications. ATC code V03AX Pharmacological properties The drug has a stimulating effect on the formation of callus and reduces pain in case of damage to peripheral nerves. Softens scar tissue and promotes its resorption Indications for use - polyneuritis - radiculitis - neuralgia - phantom pain - prevention of excessive growth of connective tissue in the early postoperative period - treatment of burns, postoperative, keloid and other extensive scars - joint contracture - keratitis, ulcers and corneal burn (in the regressive period) - to accelerate the formation of callus during fractures. Method of administration and dosage The drug is administered subcutaneously, 2 ml daily. The course of treatment for neuralgia is 8-10 days, for scars, contractures and fractures - up to 25 days. Repeating the course of treatment is recommended after one month or later. For keratitis, burns and corneal ulcers, the drug is administered subconjunctivally in a dose of 0.3-0.5 ml. The course of treatment is determined by the doctor depending on the effect of the therapy. Side effects None identified. Contraindications - infectious diseases - acute inflammatory processes - cachexia - nephritis, nephrosclerosis - liver cirrhosis - chronic heart failure - oncological diseases - pregnancy and lactation. - children under 18 years of age Drug interactions No negative effects were noted when using the drug simultaneously with other drugs. Special instructions Prescribe the drug with caution to patients with a history of allergies. Features of the effect of the drug on the ability to drive vehicles and potentially dangerous mechanisms: the drug does not affect the ability to drive vehicles and potentially dangerous mechanisms. Overdose Not identified Release form and packaging 2 ml of the drug in ampoules. 10 ampoules each along with instructions for medical use in the state and Russian languages and an ampoule scarifier in a cardboard box. When using ampoules with a break ring, packaging of ampoules without an ampoule scarifier is allowed. Storage conditions Store in a dry place at a temperature not exceeding 25 °C. Do not freeze. Keep out of the reach of children! Shelf life: 2 years Do not use after expiration date. Conditions for dispensing from pharmacies By prescription
Reviews about Vitreous humor
In most cases, reviews about the vitreous body are found on forums related to the treatment of gynecological diseases. However, many patients simply do not understand why they were prescribed this drug. Therefore, they try to consult with specialists online or ask other women who were once prescribed similar treatment.
In addition, some users are confused by the fact that they were prescribed Vitreous intramuscularly, since the instructions indicate that it is used subcutaneously. However, experts confirm that the drug can be used intramuscularly, but if such a prescription seems doubtful to patients, then it is necessary to clarify its correctness with your doctor or another doctor. Also, some women are concerned about how this drug affects the development of the fetus if pregnancy begins during treatment.
According to experts, this particular drug does not have a negative effect on either the woman’s body or the development of the fetus. But since it is usually prescribed as part of complex therapy, the effects of other drugs must also be considered. In any case, all these questions must be clarified at a doctor’s appointment, during a face-to-face consultation.
What drugs are used for intravitreal administration?
The most commonly used drugs for administration into the eye cavity are Lucentis, Ozurdex, Kenalog, Aylia and Gemaza. Antibacterial drugs can also be used for intravitreal administration.
The drug Lucentis is used to treat the wet form of age-related macular degeneration (AMD), as well as in patients with neovascular membrane (due to previous inflammation of the eye, high myopia, etc.), diabetic retinopathy, and occlusions of the central retinal vein. The action of the drug Lucentis is aimed at preventing the growth of abnormal defective vessels and associated complications in the form of multiple hemorrhages in the eye cavity, retinal detachment and loss of vision. In most cases, although not 100% of cases, Lucentis helps stop the progression of visual acuity deterioration and prevent blindness. For the same purpose, intravitreal administration of the drug Aylia is used.
Ozurdex is a drug used to treat macular edema. The dosage form of the drug Ozurdex - an implant developed for intravitreal administration - ensures long-term preservation of the therapeutic effect. Thanks to this, a prolonged, powerful anti-inflammatory and anti-edematous effect is achieved, which contributes to long-term improvement of vision.
Kenalog is a glucocorticosteroid for intravitreal administration that has an inhibitory effect on all phases of the inflammatory process in the eye cavity. In ophthalmology, the drug is used for administration parabulbarly and into the sub-Tenon's space. Kenalog is characterized by a prolonged therapeutic effect and the absence of a systemic effect on the body.
Gemaza in the form of intravitreal injections is used to treat occlusion of the main vessels of the retina, hemorrhage in the eyeball (hemophthalmos, hyphema) and in the retinal area, adhesions after surgical treatment of glaucoma. The drug provides fibrinolytic and thrombolytic effects.
Vitreous body price, where to buy
The price of Vitreous in pharmacies averages 1250-1300 rubles.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
LuxPharma* special offer
- Vitreous body in ampoules No. 10
RUR 2,400 order
show more
Pharmacy24
- Vitreous body 2 ml N10 solution TOV FZ BIOPHARMA, Ukraine / PrAT Biopharma, Ukraine
102 UAH order
PaniPharmacy
- Vitreous ampoule Vitreous solution d/in. amp. 2ml No. 10 Ukraine, Biopharma CJSC
112 UAH order
show more
Destruction of the vitreous body - symptoms and treatment
In order to better understand the mechanism of development of destruction of the vitreous body, let us turn to its structure and composition.
In its structure, the vitreous body is a gel-like mass that fills the eye from the inside and occupies approximately 2/3 of its volume. Its fixation to the inner membranes is carried out near the lens of the eye and the optic nerve head. The vitreous body contains up to 99% water, a small number of cells (hyalocytes) and protein collagen fibers. The hyaluronic acid it contains provides viscosity. There are no blood vessels in the vitreous body; metabolic processes occur due to osmosis and diffusion - the transfer of substances due to differences in concentration.
The structure of the vitreous body is heterogeneous; the following formations are distinguished in it [4]:
- central (Kloketov) canal;
- Petri channel;
- Hanover Canal;
- ligaments of Zinn;
- Vigener's ligament;
- Berger space;
- Martegiani region;
- tanks (cavities)
In terms of composition, the vitreous body is an aqueous solution of hyaluronic acid, which contains soluble proteins (albumin and globulins), inorganic phosphorus, ascorbic and citric acids and other inorganic compounds present in human blood [5].
Factors leading to the destruction of the vitreous include age-related changes, diabetes mellitus, myopia, uveitis, trauma and parasitic diseases.
Age-related changes. The pathological processes are based on metabolic disorders - a decrease in the delivery of oxygen and glucose to the cells that synthesize collagen and hyaluronic acid. At the same time, the water content in the vitreous body decreases, which leads to its wrinkling, compaction and disruption of the original structure and transparency.
The influence of diabetes mellitus. One of the complications of diabetes mellitus is the formation of strands, which not only disrupts the transparency of the vitreous body, but also causes a pulling effect on the retina, which leads to its detachment. The reason for the development of such serious disorders is the increase in the permeability of the blood-ophthalmic barrier, as a result of which the content of proteins, glucose and urea in the vitreous increases significantly. In this case, collagen fibers become compacted and become the basis for the formation of strands [4].
Myopia. With myopia, in addition to stretching the retina, light scattering circles appear due to an increase in the anterior-posterior axis of the eye - under the influence of increased light radiation, lipid peroxidation increases, which leads to a failure of biochemical and hemodynamic processes in the vitreous body. As a result, destruction occurs in the vitreous body, and foci of peripheral dystrophy occur in the retina [7].
Changes in uveitis. Clinical studies show that children with congenital uveitis have fibrosis and vitreous opacities [7]. The pathogenesis is caused by the release of proteins into the vitreous body from the surrounding choroid [8].
Injuries to the vitreous body. In case of mechanical injuries of the eyeball (for example, bruises and contusions), which are accompanied by hemorrhages into the vitreous body, the main damaging factor is the activation of free radical processes with damage to cell membranes, hypoxia, and disruption of the production of intercellular substances [9].
Parasitic diseases. Tapeworm larvae enter the eye through the bloodstream through the choroidal vessels. As the parasites grow, both vitreous opacification and inflammatory phenomena (iridocyclitis, panuveitis) are observed, which can lead to blindness [10].
Symptoms of vitreous diseases
Vitreous diseases can be congenital and inflammatory.
Congenital diseases include Norrie disease, a genetic pathology in which the retina separates and a tumor forms behind the lens.
Acquired diseases include detachment and liquefaction of the vitreous body, the appearance of hernias, hemophthalmos and various inflammations.
The main symptoms of disorders of this structure are decreased visual acuity and the appearance of floaters and flashes in the eyes (“cobwebs”, “floaters”).