Directions for use and doses
IM and IV. IV, as an infusion: ≤500 mg - over 20-30 minutes, >500 mg - 40-60 minutes. The average daily dose is 2000 mg (4 injections). The maximum daily dose is 4000 mg (50 mg/kg). The dose is adjusted taking into account the severity of the condition, body weight and renal function of the patient.
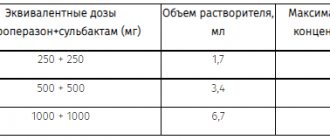
For the prevention of postoperative infections in adults, IV - 1000 mg during induction of anesthesia and 1000 mg after 3 hours. With a very high risk of postoperative infection, another 500 mg IV 8 and 16 hours after anesthesia. Children, incl. body weight less than 40 kg and for sepsis - 15 mg/kg every 6 hours (but not more than 2000 mg/day).
IM, adults - 500 or 750 mg every 12 hours (up to 1500 mg/day).
Pharmacological properties of the drug Tienam
Tienam is indicated for the treatment of mixed infections caused by strains of aerobic and anaerobic microorganisms sensitive to it. Most pathogens of mixed infections are representatives of fecal microflora, microflora of the vagina, skin or oral cavity. Bacteroides fragilis is an anaerobic pathogen, most often isolated from patients with mixed infections and, as a rule, resistant to aminoglycosides, cephalosporins and penicillins, but sensitive to Thienam. Tienam is effective in the treatment of many infections caused by aerobic and anaerobic gram-positive and gram-negative microorganisms resistant to cephalosporins, including cefazolin, cefoperazone, cephalothin, cefoxitin, cefotaxime, moxalactam, cefamandole, ceftazidime and ceftriaxone. Most infections caused by pathogens resistant to aminoglycosides (gentamicin, amikacin, tobramycin) and/or penicillins (ampicillin, carbenicillin, penicillin-G, ticarcillin, piperacillin, azlocillin, mezlocillin) are sensitive to treatment with the drug. Tienam is not indicated for the treatment of meningitis. Thienam is a broad-spectrum β-lactam antibiotic, consisting of two components: imipenem, the first representative of a new class of β-lactam antibiotics, the thienamycins, and cilastatin sodium, a specific enzyme inhibitor that blocks the metabolism of imipenem in the kidneys and significantly increases the concentration of unchanged imipenem in the urinary tract. . The weight ratio of imipenem and cilastatin sodium in Tienam is 1:1. The class of thienamycin antibiotics, to which imipenem belongs, is characterized by a wider spectrum of bactericidal action compared to any of the antibiotics already studied. Thienam is a powerful inhibitor of bacterial cell wall synthesis and has a bactericidal effect against a wide range of gram-positive and gram-negative pathogenic aerobic and anaerobic microorganisms. Like modern cephalosporin and penicillin drugs, Thienam has a broad spectrum of action against gram-negative microorganisms, but unlike others, it is the only drug that has a pronounced effect against gram-positive microorganisms, previously sensitive only to the action of earlier narrow-spectrum β-lactam antibiotics. Thienam's spectrum of action includes Pseudomonas aeruginosa, Staphylococcus aureus, Enterococcus faecalis and Bacteroides fragilis , a diverse group of problematic pathogens usually resistant to other antibiotics. Tienam is resistant to bacterial β-lactamase, which makes it effective against many microorganisms such as Pseudomonas aeruginosa Serratia spp., and Enterobacter ., which are naturally resistant to most β-lactam antibiotics. The antibacterial spectrum of action of Tienam is wider than any other antibiotic already studied, and includes almost all clinically significant pathogenic microorganisms. Microorganisms for which Tienam is usually effective in vitro include: - Gram-negative aerobic bacteria: Achromobacter ., Acinetobacter (formerly Mima-Herellea ), Aeromonas hydrophila , Alcaligenes spp., Bordetella bronchicanis, Bordetella bronchiseptica, Bordetella pertussis, Brucella melitensis , Campylobacter , Capnocytophaga spp ., Citrobacter , Citrobacter diversus, Citrobacter freundii, Eikenella corrodens . , Enterobacter aerogenes, Enterobacter agglomerans, Enterobacter cloacae, Escherichia coli, Gardnerella vaginalis, Haemophilus ducreyi, Haemophilus influenzae (including β-lactamase producing strains), Haemophilus parainfluenzae, Hafnia alvei , Klebsiella spp. (Klebsiella oxytoca, Klebsiella ozaenae, Klebsiella pneumoniae) , Moraxella spp., Morganella morganii (formerly Proteus morganii ), Neisseria gonorrhoeae (including penicillinase-producing strains), Neisseria meningitidis , Pasteurella spp., Pasteurella multocida, Plesiomonas shigelloide s , Proteus spp. ( Proteus mirabilis, Proteus vulgaris ), Providencia spp . ( Proteus alcalifaciens, Providencia rettgeri (formerly Proteus rettgeri ), Providencia stuartii ), Pseudomonas spp., including Pseudomonas aeruginosa, Pseudomonas fluorescens, Pseudomonas pseudomallei, Pseudomonas putida, P seudomonas stutzeri, Xanthomonas maltophili a (formerly Pseudomonas maltophilia ) and some strains of Pseudomonas cepacia (generally insensitive to Tienam), Salmonella spp., Salmonella typhi, Serratia spp., Serratia proteamaculans (formerly Serratia liquefaciens ), Serratia marcescens , Shigella spp., Yersinia spp . (formerly Pasteurella ) , Yersinia enterocolitica, Yersinia pseudotuberculosis ; - gram-positive aerobic bacteria: Bacillus species, Enterococcus faecalis, Erysipelothrix rhusiopathiae, Listeria monocytogenes , Nocardia , Pediococcus species, Staphylococcus aureus (including penicillinase-forming strains), Staphylococcus epidermidis (including penicillinase-forming strains), Staphylococcus saprophyticus, Streptococcus agalact iae, Streptococcus group C, Streptococcus group G, Streptococcus pneumoniae, Streptococcus pyogenes, Viridans group streptococci (including α- and γ-hemolytic strains); Enterococcus faecium and some methicillin-resistant staphylococci are insensitive to Tienam; - gram-negative anaerobic bacteria: Bacteroides ( Bacteroides distasonis (formerly Bacteroides asaccharolyticus ), Bacteroides fragilis, Bacteroides ovatus, Bacteroides thetaiotaomicron, Bacteroides uniformis, Bacteroides vulgatus ), Biophilia wadsworthia , Fusobacterium ( Fusobacterium necrophorum, Fusobacterium n ucleatum ), Porphyromonas asaccharolytica (formerly Bacteroides asacchrolyticus ), Prevotella bivia (formerly Bacteroides bivius ), Prevotella disiens (formerly Bacteroides disiens ), Prevotella intermedia (formerly Bacteroides vulgatus intermedius ), Prevotella melaninogenica (formerly Bacteroides melaninogenicus ), Veillonella spp .; - gram-positive anaerobic bacteria: Actinomyces , Bifidobacterium , Clostridium , Clostridium perfringens , Eubacterium Lactobacillus species, Mobiluncus species , Microaerophilic streptococcus , Peptococcus species , Peptostreptococcus , Propionibacterium (including P. acnes ); - others: Mycobacterium fortuitum, Mycobacterium smegmatis. in vitro studies indicate that imipenem has a synergistic effect with aminoglycoside antibiotics against some isolates of Pseudomonas aeruginosa . In healthy volunteers, intravenous infusion of Thienam at a dose of 500 mg over 20 minutes was accompanied by peak plasma levels of imipenem ranging from 21 to 58 mcg/ml. The half-life of imipenem in blood plasma was 1 hour. About 70% of the administered antibiotic was detected unchanged in the urine within 10 hours; further excretion of the drug in the urine was not observed. When using the drug Tienam according to the schedule every 6 hours, no accumulation of imipenem in the blood plasma or urine was detected in patients with normal renal function. Co-administration of Tienam and probenecid resulted in minimal increases in plasma levels and half-life of imipenem. When used alone, imipenem is metabolized in the kidneys by dihydropeptidase-1. Individual urinary recovery ranges from 5–40%, with an average of 15–20% in several studies. The binding of imipenem to human serum proteins is about 20%. Cilastatin, a specific inhibitor of the enzyme dehydropeptidase-1, effectively inhibits the metabolism of imipenem, so the simultaneous use of imipenem and cilastatin allows one to achieve therapeutic antibacterial levels of imipenem in urine and blood plasma. Peak cilastatin levels following a 20-minute IV infusion of Tienam 500 mg ranged from 21 to 55 mcg/mL. The half-life of cilastatin from the blood plasma is about 1 hour. About 70–80% of the dose of cilastatin is excreted unchanged in the urine within 10 hours after administration of the drug Tienam. After this, cilastatin is not detected in the urine. About 10% was determined as a metabolite of N-acetyl, which has an inhibitory effect on dehydropeptidase, which is comparable to that of the parent drug cilastatin. The combined use of Tienam and probenecid led to a 2-fold increase in plasma levels and an increase in the half-life of cilastatin, but did not affect the recovery of cilastatin in the urine. The binding of cilastatin to human serum proteins is about 40%.
Interactions of the drug Tienam
In patients receiving ganciclovir and Tienam simultaneously for intravenous infusion, generalized seizures were detected. These drugs should not be coadministered unless the expected benefit outweighs the potential risk. During post-marketing studies, decreased plasma levels of valproic acid have been reported when administered concomitantly with carbapenems, and in some cases, sudden onset seizures have been reported. When imepenem is used concomitantly with valproic acid, it is necessary to carefully monitor the level of valproic acid in the blood plasma.
Side effects of the drug Thienam
Tienam is generally well tolerated. Adverse reactions rarely require discontinuation of treatment and are usually mild and transient; Severe side effects are rare. Among the known side effects, local ones are most often observed. Local manifestations (the same for different routes of administration): erythema, pain and infiltrates at the sites of drug administration, thrombophlebitis. Allergic reactions/skin manifestations : anaphylactic reactions, rash, pruritus, urticaria, erythema multiforme, Stevenson-Johnson syndrome, angioedema, toxic epidermal necrolysis (rare), exfoliative dermatitis (rare), candidiasis, fever (including drug-induced fever). Reactions from the gastrointestinal tract : nausea, vomiting, diarrhea, pigmentation of teeth and/or tongue. As with almost all other broad-spectrum antibiotics, pseudomembranous colitis may occasionally occur. Reactions from the hematopoietic system: eosinophilia, leukopenia, neutropenia, including agranulocytosis, thrombocytopenia, thrombocytosis, decreased hemoglobin levels, pancytopenia and increased prothrombin time. Some patients may have a positive direct Coombs test. Reactions from liver function: increased levels of transaminases, bilirubin and/or alkaline phosphatase; liver failure (rare), hepatitis (rare) and fulminant hepatitis (very rare). Reactions from renal function: oliguria/anuria, polyuria, acute renal failure (rare). It is difficult to assess whether Thienam affects renal function, since, as a rule, other factors predisposing to impaired renal function and the development of azotemia are simultaneously present. Increases in serum creatinine and blood urea nitrogen levels were detected. A change in the color of the urine has been noted, which does not pose any threat and should not be confused with hematuria. Reactions from the nervous system: when using the drug Tienam, like other β-lactam antibiotics, side effects such as myoclonus, mental disorders (including hallucinations), confusion and convulsions, paresthesia, and encephalopathy develop. Reactions from the senses: hearing loss, change in taste. Patients with granulocytopenia Nausea and/or vomiting caused by the use of Tienam occurs more often in patients with granulocytopenia than without it.