Cetirizine, drops
Dosage regimen
Adults and children over 12 years old
The recommended dose is 10 mg (24 drops) once daily.
Children 6-12 years old
The recommended dose is 5 mg (12 drops) twice daily.
Children 2-5 years old
The recommended dose is 2.5 mg (6 drops) twice daily.
Children from 1 year to 2 years
The recommended dose is 2.5 mg (6 drops) once or twice daily.
Children from 6 months to 1 year
The recommended dose is 2.5 mg (6 drops) once daily.
The maximum duration of treatment in children with seasonal rhinitis should not exceed 4 weeks.
Special patient groups
Elderly
According to available data, with normal renal function, age is not a reason to reduce the dose.
Patients with impaired renal function
There is no data confirming the effectiveness/safety of the drug in patients with impaired renal function. Since cetirizine is mainly excreted through the kidneys (see section “Pharmacokinetic properties”), if it is impossible to use alternative treatment, the dose of the drug should be selected individually, taking into account renal function. The table below helps you choose the right dose. To use this table, it is necessary to determine creatinine clearance (CC) in ml/min. To do this, it is necessary to determine the serum creatinine level and calculate CC using the following formula:
CC = [140 - age (in years)] x weight (kg) (x 0.85 for women)
72 x serum creatinine (mg/dL)
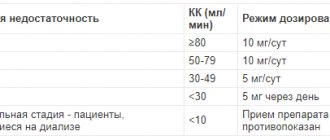
Dose adjustment in patients with impaired renal function:
| Group | CC (ml/min) | Dose and frequency of administration |
| Normal kidney function | >80 | 10 mg (24 drops) 1 time/day |
| Mild renal impairment | 50-79 | 10 mg (24 drops) 1 time/day |
| Moderate renal impairment | 30-49 | 5 mg (12 drops) 1 time/day |
| Severe renal impairment | 10-29 | 5 mg (12 drops) once every two days |
| End stage renal failure, dialysis | <10 | Contraindicated |
For children with impaired renal function, the dose should be selected individually, taking into account renal clearance, as well as the age and body weight of the patient.
Patients with impaired liver function
In patients with only liver dysfunction, there is no need for dose adjustment.
Patients with impaired liver and kidney function
Dose adjustment is recommended (see section “Patients with impaired renal function” above).
Mode of application
Drops for oral administration.
The use of the drug does not depend on food intake (see section “Pharmacokinetic properties”).
The solution should either be dripped into a spoon or diluted with water and taken orally. If the solution is diluted with water, then it should be taken into account that the volume of liquid used to dissolve the drug corresponds to the amount of liquid that the patient is able to drink. This is especially important when dosing the drug to children.
Cetirizine drops orally 10 mg/ml 20 ml (Ozone)
Due to the potential depressant effect on the central nervous system, caution should be exercised when prescribing the drug to children under 1 year of age who have the following risk factors for sudden infant death syndrome, such as (but not limited to): - sleep apnea syndrome or sudden infant death syndrome death of infants of a brother or sister; - maternal drug abuse or smoking during pregnancy; - young age of mother (19 years and younger); - smoking abuse by a nanny caring for a child (one pack of cigarettes a day or more); - children who regularly fall asleep face down and are not placed on their back; - premature (gestational age less than 37 weeks) or low birth weight (below the 10th percentile of gestational age) children; - when taking drugs together that have a depressing effect on the central nervous system. The drug contains excipients methyl parahydroxybenzoate and propyl parahydroxybenzoate, which can cause allergic reactions, including delayed ones. In patients with spinal cord injury, prostatic hyperplasia, or other predisposing factors to urinary retention, caution is required as cetirizine may increase the risk of urinary retention. Caution is recommended when using cetirizine concomitantly with alcohol, although no clinically significant interaction with alcohol was observed at therapeutic doses (at a blood alcohol concentration of 0.5 g/l). Caution should be observed in patients with epilepsy and increased convulsive readiness. Before prescribing allergy tests, a three-day “washing out” period is recommended due to the fact that H1-histamine receptor blockers inhibit the development of skin allergic reactions. Effect of the drug on the ability to drive vehicles and operate machinery: An objective assessment of the ability to drive vehicles and operate machinery did not reliably reveal any adverse events when taking the drug at the recommended dose. However, for patients with symptoms of drowsiness while taking the drug, it is advisable to refrain from driving a car, engaging in potentially hazardous activities, or operating machinery that requires increased concentration and speed of psychomotor reactions.
Cetirizine
Description of selected adverse reactions:
Cases of pruritus, including intense itching and/or urticaria, have been reported following discontinuation of cetirizine.
If you experience the side effects listed in the instructions, or they get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
Overdose
Symptoms observed after apparent drug overdose have affected the central nervous system or have been associated with a possible anticholinergic effect. Symptoms that occurred after taking at least five times the recommended daily dose included the following: confusion, diarrhea, fatigue, headache, malaise, mydriasis, itching, restlessness, sedation, somnolence, stupor, tachycardia, tremor, urinary retention.
Treatment:
There is no specific antidote.
In case of overdose, symptomatic or supportive treatment is recommended. Gastric lavage and/or activated charcoal may be effective if the overdose has occurred recently. Cetirizine is partially eliminated by dialysis.
Interaction with other drugs
Concomitant use with azithromycin, cimetidine, erythromycin, ketaconazole or pseudoephedrine does not affect the pharmacokinetic parameters of cetirizine.
No pharmacokinetic interactions were observed. in vitro test
, cetirizine does not affect the protein binding effect of warfarin. Concomitant use of azithromycin, erythromycin, ketoconazole, theophylline and pseudoephedrine did not reveal significant changes in clinical laboratory parameters, vital signs and ECG.
In a study of co-administration of theophylline (400 mg per day) and cetirizine (20 mg per day), a small but statistically significant increase in 24-hour AUC (area under the curve) was found by 19% for cetirizine and 11% for theophylline. In addition, maximum plasma levels increased to 7.7% and
6.4%, respectively, for cetirizine and theophylline. At the same time, the clearance of cetirizine decreased by -16%, and also by -10% in the case of theophylline, when cetirizine was taken by patients who had previously received treatment with theophylline. However, pretreatment with cetirizine did not significantly affect the pharmacokinetic parameters of theophylline.
Cetirizine instructions
The regimen for taking an antihistamine directly depends on its release form and the age of the patient. The daily dosage may vary in cases of decreased renal function or renal failure.
Let's consider general recommendations for use:
- Adults are recommended to take one tablet (10 mg) once a day or half a tablet (5 mg) twice a day.
- Children over 6 years of age can take the drug in the same way.
Take the tablets with a sufficient volume of water, at least 200 ml.
The duration of treatment, as well as the dosage, depends on the individual characteristics of the patient and the nature of the allergy. In cases with seasonal exacerbations, the treatment period reaches 6 weeks of continuous use of the drug based on cetirizine [2].
Cetirizine FT
- Pharmacodynamics Cetirizine is a piperazine derivative and is a carboxylated metabolite of hydroxyzine. Cetirizine has selective antagonism of peripheral H1 receptors and has a pronounced antiallergic effect. Has antipruritic and antiexudative effect. It has almost no effect on other types of receptors, does not have anticholinergic and antiserotonergic effects. Cetirizine affects the “early” histamine-dependent stage of the allergic reaction, and also limits the release of inflammatory mediators at the “late” stage of the allergic reaction, and has an anti-inflammatory effect. It reduces the migration of eosinoyls, neutrophils and basophils, stabilizes the membranes of mast cells. Reduces capillary permeability, prevents the development of tissue edema, and eliminates spasm of smooth muscles. Eliminates skin reaction to the introduction of histamine, specific allergens, cooling, reduces histamine-induced bronchial obstruction in mild bronchial asthma. Cetirizine is less lipophilic compared to hydroxyzine, practically does not penetrate the blood-brain barrier and does not act on H1 receptors in the central nervous system. In therapeutic doses it does not have a sedative effect. After taking 10 mg of cetirizine, the effect develops within 20 minutes in 50% of patients, after 60 minutes in 95% of patients and lasts more than 24 hours. With repeated doses, addiction to the drug does not develop. After discontinuation of treatment, the effect persists for three days.
Pharmacokinetics Absorption: After oral administration, cetirizine is rapidly and completely absorbed from the gastrointestinal tract. Cmax in blood plasma is reached within 1.0±0.5 hours and is approximately 300 ng/ml. When taken simultaneously with food, the completeness of absorption does not change, but its rate decreases. The equilibrium state is achieved on the third day. In volunteers, pharmacokinetic parameters such as Cmax and AUC are unimodal. The degree of bioavailability is similar when cetirizine is used in the form of a solution, tablets or capsules. In the dose range from 5 to 60 mg, the pharmacokinetics are linear.
Distribution: In adults, after taking 10 mg of the drug orally and in children after taking 5 mg of the drug, the volume of distribution is approximately 35 l (0.5 l/kg) and 17 l, respectively. The binding of cetirizine to plasma proteins is 93±0.3%. Cetirizine does not alter the binding of warfarin to blood proteins. When taking 10 mg/day for 10 days, no accumulation of the drug was observed.
Metabolism: Cetirizine does not undergo first-pass metabolism. In small quantities, it is metabolized in the liver by O-dealkylation to form a pharmacologically inactive metabolite (unlike other H1-histamine receptor blockers, which metabolize with the participation of the cytochrome P 450 system).
Excretion: About 2/3 of the administered dose of cetirizine is excreted unchanged from the body in the urine. The half-life in adults is about 10 hours, in children 6-12 years old - about 6 hours, in children aged 2-6 years - 5 hours, from 6 months to 2 years - 3.1 hours. After taking 10 mg of the drug in adults, total clearance increases to 0.6 ml/min/kg. in children after taking 5 mg - up to 0.93 ml/min/kg. After discontinuation of cetirizine, its blood level quickly decreases to undetectable low values.
In elderly patients and patients with chronic liver dysfunction, after a single dose of cetirizine at a dose of 10 mg, T1/2 increases by approximately 50%, and clearance decreases by 40% compared to middle-aged patients. In patients with mild renal impairment (creatinine clearance > 40 ml/min), the pharmacokinetic parameters of cetirizine are similar to those in healthy volunteers. In patients with moderate renal failure and in patients. patients on hemodialysis (creatinine clearance < 7 ml/min), a single dose of 10 mg of the drug led to a threefold prolongation of T1/2 and a decrease in clearance by 70%, which requires dose adjustment. Cetirizine is not removed from the body by hemodialysis.
Cetirizine Sandoz, 10 mg/ml, oral drops, 20 ml, 1 pc.
Data obtained from clinical studies
The results of clinical studies have demonstrated that the use of cetirizine in recommended doses leads to the development of minor undesirable effects on the central nervous system, including drowsiness, fatigue, dizziness and headache. In some cases, paradoxical stimulation of the central nervous system has been reported.
Despite the fact that cetirizine is a selective blocker of peripheral H1 receptors and has virtually no anticholinergic effect, isolated cases of difficulty urinating, disturbances of accommodation and dry mouth have been reported.
Liver dysfunction has been reported, accompanied by increased levels of liver enzymes and bilirubin. In most cases, adverse events resolved after discontinuation of cetirizine.
List of unwanted side reactions. There is data from double-blind, controlled clinical trials comparing cetirizine with placebo or other antihistamines used at recommended doses (10 mg once daily for cetirizine) in more than 3200 patients, on which a reliable analysis can be made safety data.
According to the results of the pooled analysis, in placebo-controlled studies with cetirizine 10 mg (n=3260) and placebo (n=3061), the following adverse reactions were identified with an incidence of 1% or higher.
General disorders and disorders at the injection site: fatigue - 1.63 and 0.95%.
From the nervous system: dizziness - 1.1 and 0.98%; headache - 7.42 and 8%.
From the gastrointestinal tract: abdominal pain - 0.98 and 1.08%; dry mouth - 2.09 and 0.82%; nausea - 1.07 and 1.14%.
From the mental side: drowsiness - 9.63 and 5%.
From the respiratory system, chest and mediastinal organs: pharyngitis - 1.29 and 1.34%.
Although the incidence of somnolence in the cetirizine group was higher than that in the placebo group, most cases were mild or moderate in severity. When objectively assessed in other studies, it was confirmed that the use of cetirizine at the recommended daily dose in healthy young volunteers does not affect their daily activities.
Children. In placebo-controlled studies in children aged 6 months to 12 years, the following adverse reactions were identified with an incidence of 1% or higher in the cetirizine (n=1656) and placebo (n=1294) groups.
From the gastrointestinal tract: diarrhea - 1 and 0.6%.
From the mental side: drowsiness - 1.8 and 1.4%.
From the respiratory system, chest and mediastinal organs: rhinitis - 1.4 and 1.1%.
General disorders and disorders at the injection site: fatigue - 1 and 0.3%.
Post-registration experience
In addition to the adverse events identified during clinical trials and described above, the following adverse reactions were observed during post-marketing use of cetirizine.
Adverse events are presented below by MedDRA organ system class and incidence, based on data from post-marketing use of cetirizine.
The incidence of adverse events was determined as follows: very often (≥1/10); often (≥1/100,
From the blood and lymphatic system: very rarely - thrombocytopenia.
From the immune system: rarely - hypersensitivity reactions; very rarely - anaphylactic shock.
Metabolic and eating disorders: frequency unknown - increased appetite.
From the mental side: infrequently - agitation; rarely - aggression, confusion, depression, hallucinations, sleep disturbance; very rarely - tic; frequency unknown - suicidal ideation.
From the nervous system: infrequently - paresthesia; rarely - convulsions; very rarely - taste perversion, dyskinesia, dystonia, fainting, tremor; frequency unknown - memory impairment, incl. amnesia.
From the organ of vision: very rarely - disturbance of accommodation, blurred vision, nystagmus.
On the part of the hearing organs: frequency unknown - vertigo.
From the cardiovascular system: rarely - tachycardia.
From the digestive system: infrequently - diarrhea.
Hepatobiliary disorders: rarely - changes in liver function tests (increased activity of transaminases, alkaline phosphatase, gamma-glutamyl transferase and bilirubin levels).
On the skin: infrequently - rash, itching; rarely - urticaria; very rarely - angioedema, persistent drug erythema.
From the urinary system: very rarely - dysuria, enuresis; frequency unknown - urinary retention.
General disorders: infrequently - asthenia, malaise; rarely - peripheral edema.
Research: rarely - weight gain.