Similar drugs
- Avonex lyophilisate i/m
Biogen Aidek B.V. Netherlands - Avonex solution w/o
Biogen Aidek B.V. Netherlands
- Genfaxon SC solution
Laboratory Tutor S.A.S.I.F.I.A Argentina
- Rebif SC solution
Merck Serono S.A. Switzerland
- SinnoVex lyophilisate solution IM
Sia AFS, LLC Russia
- Teberif SC solution
BIOCAD, JSC Russia
Pharmacodynamics:
SinnoVex - recombinant Interferon beta - 1a is obtained using recombinant DNA technology on Chinese hamster ovary cells with an integrated human interferon beta genome. It is a glycolyzed polypeptide containing 166 amino acids with a molecular weight of 22,500.
The amino acid sequence corresponds to natural human interferon beta.
Interferons are natural proteins produced by eukaryotic cells in response to viral infection and other biological factors. Interferons are cytokines that are mediators of the antiviral, antiproliferative and immunomodulatory systems of the body.
Interferon beta is synthesized by various types of cells, including fibroblasts and macrophages. Natural interferon beta and the drug SinnoVex (interferon beta-1a) exist in glycosylated form and contain a single complex hydrocarbon fragment linked to the N atom. Glycosylation of proteins affects their stability, activity, biodistribution and half-life.
The biological properties of SinnoVex are determined by its ability to bind to specific receptors on the surface of cells of the human body and trigger a complex cascade of intercellular interactions, leading to interferon-mediated expression of numerous gene products and markers, such as major histocompatibility complex class I, Mx protein, 2'/5' -oligoadenylate synthetase, b2-microglobulin and neopterin. The presence of some of these compounds was detected in the serum and cellular blood fractions of patients receiving SinnoVex. After intramuscular administration of one dose of the drug, the serum levels of these compounds remained elevated for at least 4-7 days. It is unknown whether the mechanism of action of the drug SinnoVex in the treatment of multiple sclerosis is related to the triggering of the biological interactions described above, since the pathophysiology of multiple sclerosis has not yet been sufficiently studied. The effect of the drug SinnoVex in the treatment of multiple sclerosis was demonstrated in a double-blind study conducted on patients suffering from a relapsing form of multiple sclerosis (for the drug "SinnoVex" n = 41, for the comparison drug "Avonex" n = 43). In accordance with the study design, patients were monitored for different periods of time. 60 study participants took the drug "SinnoVex" under supervision for 2 years. It was shown that the total number of patients who experienced progression of disability (as determined by the Kaplan-Meier table) at the end of the second year was 34% with Avonex and -32% with SynnoVex. Progression of disability was measured as a 1.0 point increase in disability score (EDSS) that persisted for at least 6 months. It was also found that the use of the drug SinnoVex led to a reduction in the frequency of relapses within a year by one third. These data were obtained after treatment lasting more than one year.
Other manifestations
In addition, the following negative manifestations from the drug "SinnoVex" may occur:
- Research: increased concentration of urea nitrogen in the blood, increased concentration of potassium in the blood, decreased hematocrit, neutropenia, leukopenia, lymphocytopenia, thrombocytopenia, changes in liver function test parameters, increase or decrease in body weight.
- Vascular and cardiac system: facial flushing, vasodilation, tachycardia, arrhythmia, pulsation, congestive heart failure, cardiomyopathy.
- Endocrine system: hyperthyroidism, hypothyroidism, thrombocytopenia, pancytopenia.
- Nervous system: headache, hyperesthesia, muscle spasms, migraine, convulsions, paresthesia, dizziness, hypertension, fainting, neurological symptoms.
- Respiratory system: shortness of breath, rhinorrhea.
- Digestive system: nausea, diarrhea, vomiting, anorexia, autoimmune hepatitis, hepatitis, liver failure.
- Skin, subcutaneous tissues: bruises, increased sweating, rash, exacerbation of psoriasis, urticaria, vesicular rash, itching, angioedema, alopecia.
- Bone and muscle system: skeletal muscle stiffness, muscle stiffness, back pain, limb pain, arthralgia, myalgia, neck pain, muscle spasms, arthritis, muscle weakness, systemic lupus erythematosus.
- Local reactions: burning at the injection site, night sweats, malaise, fatigue, pain, asthenia, hematoma at the injection site, erythema, chest tenderness, bleeding at the injection site, necrosis at the injection site, inflammatory reaction, cellulite, abscess.
- Immune system: hypersensitivity reactions - itching, rash, urticaria, shortness of breath, angioedema, anaphylactic shock, anaphylactic reaction.
- Reproductive system: menorrhagia, metrorrhagia.
- CNS: insomnia, depression, emotional lability, confusion, anxiety, psychosis, suicide.
The above reactions that occur at the site of drug administration may, in some cases, require surgical intervention (abscess, necrosis).
Indications:
— SinnoVex is intended for the treatment of patients suffering from relapsing multiple sclerosis (MS), characterized by at least two relapses over the previous three-year period with no evidence of disease progression between relapses. SinnoVex slows the progression of disability and increases the interval between relapses by more than two years.
- Treatment of patients who have had a case of demyelination as a result of an active inflammatory process requiring intravenous corticosteroids, when a diagnosis other than multiple sclerosis has been excluded.
Contraindications:
- Known hypersensitivity to natural or recombinant interferon beta, human serum albumin or any other component of the drug;
— period of pregnancy and lactation;
- severe depression
- severe depression and the appearance of suicidal thoughts;
- patients with epilepsy with seizures that are difficult to control with medications;
- age up to 12 years due to the lack of clinical data on the use of the drug in this group.
Drug interactions
The interaction of SinnoVex (interferon beta-1a) with other drugs, including corticosteroids or adrenocorticotropic hormone (ACTH), has not been studied in humans. At the same time, the experience of clinical trials shows that when treating MS during an exacerbation of the disease, SinnoVex can be taken with corticosteroids or ACTH.
The ability of interferons to reduce the activity of enzymes of the cytochrome P450 family is known. Therefore, the administration of SinnoVex simultaneously with drugs whose clearance depends largely on the cytochrome P450 system (antidepressants, antiepileptic drugs) requires caution.
Carefully:
SinnoVex, like other interferons, should be used with caution when treating patients suffering from depression or depressive disorders. It is known that when using interferons, depression and suicidal thoughts may occur, and in the group of people suffering from multiple sclerosis, the frequency of such phenomena increases. The occurrence of depressive states is possible at any time during treatment with SinnoVex. If any signs of depression or suicidal thoughts occur, patients should immediately contact their doctor. Such patients must be closely monitored during treatment and, if necessary, appropriate therapeutic measures must be urgently applied. In some cases, it may be necessary to stop using the drug.
Caution is required when prescribing SinnoVex to patients who have previously suffered from seizures. In the case of patients who have not previously suffered from epileptic seizures, as well as those taking anti-epileptic drugs, in particular if the treatment of epilepsy and the use of anti-epileptic drugs are not controlled or are inadequately controlled.
Caution should be exercised when prescribing, as well as careful monitoring of patients suffering from severe renal and liver failure, as well as suppression of bone marrow hematopoiesis.
When using interferon beta, signs of liver dysfunction were observed, such as an increase in the level of liver enzymes in the blood serum, the development of hepatitis, including autoimmune, and liver failure. However, it is not known whether this is due to the use of interferon beta-1a or due to the use of other drugs that are usually prescribed for such
patients. Patients should be carefully monitored for signs of liver dysfunction, especially if interferon is used in conjunction with other hepatotoxic drugs.
When using the drug SinnoVex, the condition of patients with diseases of the cardiovascular system should be carefully monitored: angina pectoris, previous myocardial infarction, decompensated heart failure, arrhythmia. Manifestations of influenza-like syndrome caused by the use of the drug can have a stressful effect on such patients.
When using interferons, deviations in laboratory parameters occur, therefore, in addition to the usual laboratory tests performed by patients with MS, during treatment it is recommended to perform a count of blood cells (including platelets), determination of the leukocyte formula and a biochemical blood test (including liver enzymes). Those patients who have signs of bone marrow suppression may require a more thorough blood test with determination of cellular elements by fraction and platelets.
When using SinnoVex, interferon-neutralizing antibodies may appear in the blood serum, which can reduce the activity of interferon beta-1a, and therefore the clinical effectiveness of the drug. Available data indicate that after 12 months of treatment, antibodies to interferon beta-1a appear in the serum of approximately 8% of patients.
Negative Impacts
According to reviews of SinnoVex, interferons most often cause a flu-like syndrome as a negative symptom. It manifests itself by the development of nausea, headache, chills, fever, muscle pain, feelings of fatigue, and weakness. These symptoms are usually more pronounced at the beginning of therapy, and their frequency decreases as it continues.
In order to alleviate negative manifestations, the use of antipyretic analgesics is allowed, which are recommended to be taken before the administration of SinnoVex, and then every 6 hours for 24 hours after each injection. Before using any medication in conjunction with therapy, you should consult a doctor. In cases where a specialist recommends taking antipyretic analgesics, it is important to carefully follow the recommendations. It is forbidden to increase the dosage of recommended drugs.
According to the instructions and reviews of SinnoVex, at any stage of therapy, neurological symptoms similar to exacerbation of multiple sclerosis may occur: alternating episodes of muscle weakness and muscle hypertonicity that limit voluntary movements. These episodes are limited, as a rule, in time, occur after administration of the drug, and can be repeated with repeated use of the drug. In some cases, these symptoms may be accompanied by a flu-like syndrome.
Pregnancy and lactation:
Due to the potential risk of adverse reactions, the use of SinnoVex during pregnancy is contraindicated. Available evidence suggests spontaneous abortion. Due to the potential risk of adverse reactions in an infant, SinnoVex is contraindicated for use during lactation.
Women with preserved reproductive capacity should use effective methods of contraception. If pregnancy occurs or pregnancy is planned during treatment with SinnoVex, the patient should be informed of the potential danger and the advisability of discontinuing treatment should be considered. In patients with a high rate of relapses before treatment, the risk of severe relapses due to discontinuation of SinnoVex in case of pregnancy should be weighed against the possible increased risk of spontaneous abortion due to its use during pregnancy.
Reviews about SinnoVex
Drugs for the treatment of MS are included in the most expensive state procurement program “7 high-cost nosologies”, under which all patients are provided with drugs from the federal budget. In reviews of SinnoVex, a generic that replaced the original drug manufactured in the USA in the purchase, many patients express dissatisfaction with this replacement. They indicate that when using SinnoVex, the temperature increases more often, convulsions appear, causing physical pain; these adverse reactions significantly worsen the quality of life. Due to intolerance to therapy, some are forced to refuse it. There is evidence that the fraction of interferon beta-1a in SinnoVex is significantly reduced, and other protein compounds (mainly human serum albumin) are contained in increased quantities. This indicates a low degree of purification of the drug, a possible discrepancy between the dose of interferon in the vial and the declared 30 mcg, and the low quality of the drug.
At the same time, it really helps other patients who use SinnoVex. It softens relapses and exacerbations or completely eliminates them, reduces old foci of the disease and prevents the formation of new ones. Associated adverse reactions, such as flu-like syndrome, depression, headaches (increased on the day of injection), are considered moderate by these patients.
Directions for use and dosage:
The use of the drug should be initiated under the guidance and supervision of a physician experienced in the treatment of multiple sclerosis ( MS ).
Adults: The recommended dose of SinnoVex (interferon beta-1a) for RMS is 30 mcg (6 million IU), i.e. 1 ml of dissolved drug in a bottle, and is administered intramuscularly once a week.
Treatment can be started either with a full dose of 30 mcg or with a half dose once a week and gradually increased to a full dose of 30 mcg so that the body can get used to the drug. To achieve sufficient effectiveness after the initial period of treatment, it is necessary to increase the dose to 30 mcg once a week and then maintain this dose.
A higher dose (60 mcg) once weekly does not provide additional benefit.
Children and adolescents: Safety profile when prescribing the drug
SinnoVex 30 mcg intramuscularly once a week in adolescents 12-16 years of age is similar to the safety profile for adults. There is no information on the use of the drug in the treatment of children under 12 years of age, therefore SinnoVex should not be used in this patient population.
Elderly patients: Clinical studies did not include a sufficient number of patients over 65 years of age to determine whether there was a possible difference in response to treatment in this age group compared with younger patients. However, based on the clearance of the active substance, there is no theoretical basis for adjusting the dose of this drug in elderly patients.
Injections of the drug should, if possible, be made at the same time and on the same day of the week. The intramuscular injection site should be changed every week.
The duration of the course of therapy is determined individually. After two years of treatment, the patient should undergo a clinical examination and, on an individual basis, the attending physician may recommend continuing the course of therapy. Treatment should be discontinued if the patient develops chronic progressive MS.
It is possible for the patient to perform injections with the permission of the attending physician and after training in the method of intramuscular injections.
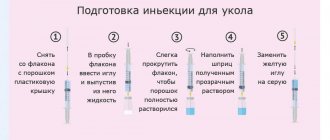
The following recommendations are intended for those who inject the drug themselves:
— Each package contains four trays with single doses of the drug each, and eight antiseptic wipes soaked in alcohol in separate hermetically sealed bags. Each tray includes a bottle with lyophilized SinnoVex powder, an ampoule with water for injection 1 ml, a syringe with a needle for preparing the solution and a needle for injection.
- Remove the drug from the refrigerator and leave at room temperature 15 - 30 ° C for about 30 minutes. Do not use external heat sources, such as hot water, to heat the solvent ampoule.
— After washing your hands, place two antiseptic wipes and a dose tray on a clean surface. Carefully open the tray packaging and remove the contents. It is advisable to additionally prepare sterile medical cotton wool, a disinfectant solution (for example, ethyl alcohol 70%) and a bactericidal adhesive plaster.
— Open the packages of the syringe and needle. Without removing the protective cap from the needle, put the needle on the syringe, turning it half a turn. — Using an alcohol wipe, open the ampoule of water for injection, breaking the ampoule along the red line on the neck. Remove the cover from the needle without rotating the needle and draw water for injection into the syringe. Leave the protective cap for future use. If air bubbles get into the syringe, carefully remove them by holding the syringe vertically with the needle and gently pressing the piston. Place the protective cap on the needle.
— Remove the cap from the bottle with the drug. Wipe the top of the bottle with the drug with an alcohol wipe.
— Remove the protective cap from the needle, pierce the rubber stopper of the bottle with the drug with the needle. Direct the needle to the side wall of the bottle and slowly inject the solvent (the entire contents of the syringe).
- Leaving the needle and syringe in place, carefully rotate the contents of the bottle until all the powder has dissolved. Do not shake the bottle vigorously as this will cause foaming. If the solution is cloudy or colored, or if solid particles are visible in the solution, the vial should not be used. A light yellow color of the solution is acceptable.
— Before withdrawing the solution, completely immerse the piston in the syringe to remove air. Next, place the bottle on the work surface at a slight angle. The entire needle should be in the vial, and the end of the needle should be constantly immersed in the solution. Slowly withdraw the solution into the syringe to the 1 ml mark located on the side surface of the syringe. Remove the syringe with the needle from the bottle. Place a protective cap on the needle. Rotate to disconnect the needle from the syringe. Do not touch the outlet of the syringe!
— The second needle is intended for administering the SinnoVex solution. It is a standard needle for intramuscular injections. In the same way as described above, twist the needle onto the syringe. Remove the plastic protective cap from the needle and set it aside. To remove air, turn the syringe upside down with the needle and gently tap it so that the bubbles collect at the top. Gently press the plunger to remove air, allowing only a small drop of solution to escape. Put the protective cover in place and put the syringe aside while preparing the injection site.
— Clean the selected injection site with a new alcohol wipe. Remove the plastic protective cover from the needle and insert the needle through the skin into the muscle tissue. Perform the injection slowly, then remove the needle and syringe. Wipe the injection site with an alcohol wipe and, if necessary, cover the injection site with a band-aid.
— The subsequent injection must be done in another place on the body.
- If circumstances arise that do not allow an injection, when the solution has already been prepared, you can place it for no more than 5.5 - 6 hours in the refrigerator at a temperature of 2-8 ° C, after which, having previously brought the solution temperature to room temperature, make the injection according to the above scheme.
The prepared solution, stored for more than 6 hours in the refrigerator or left at room temperature for more than 30 minutes, is unsuitable for further use.
Elderly patients
A sufficient number of patients over 65 years of age were not included in clinical trials, so the likely differences in response between these patients and younger patients have not been established. If we take into account the clearance of the active component, there are no theoretical grounds for adjusting the dose when treating elderly people.
If possible, injections should be given on the same day of the week, at the same time of day. It is recommended to change the intramuscular injection sites every time.
This is confirmed by the instructions for use for SinnoVex.
Package:
Lyophilisate for the preparation of a solution for intramuscular administration of 30 mcg (6 million ME) in a 3 ml transparent colorless neutral glass bottle, closed with a rubber stopper, an aluminum cap and a plastic cap with tamper evident.
Solvent 1 ml in a 2 ml colorless neutral glass ampoule.
One bottle of lyophilisate, one ampoule of solvent, one disposable empty syringe and 2 needles are packaged in a sealed plastic tray.
4 plastic trays and 8 alcohol wipes are placed in a cardboard box along with instructions for use.
In order to protect the packaging from counterfeiting, 2 protective stickers with the logo and the inscription “CinnaGen QA Approved CinnaGen” are affixed to the cardboard box.
Price for SinnoVex in pharmacies
Since the drug is included in the list of Vital and Essential Drugs (Vital and Essential Medicines), there is a registered fixed price for SinnoVex, which is for a sealed polymer package with four sets, each of which contains a bottle with lyophilisate (30 mcg) + an ampoule with solvent (1 ml) + disposable syringe + disposable needles (2 pcs.) + alcohol wipes (2 pcs.), – RUB 15,645.45.
The cost of SinnoVex in some online pharmacies for a package of four sets varies from 7,300 to 11,500 rubles.