Release form, packaging and composition of the drug Cefoperazone + Sulbactam
Powder for the preparation of a solution for intravenous and intramuscular administration, white or white with a yellowish tint; the reconstituted solution is light yellow to yellow.
| 1 fl. | |
| cefoperazone sodium | 518 mg, |
| which corresponds to the content of cefoperazone | 500 mg |
| sulbactam sodium | 550 mg, |
| which corresponds to the content of sulbactam | 500 mg |
1 g - Glass bottles with a capacity of 10 ml (1) - cardboard packs. 1 g - Glass bottles with a capacity of 10 ml (5) - cardboard packs. 1 g - Glass bottles with a capacity of 10 ml (10) - cardboard packs. 1 g - Glass bottles with a capacity of 10 ml (1) complete with solvent - water for 5 ml ampoules - 1 amp. - cardboard packs. 1 g - Glass bottles with a capacity of 10 ml (5) complete with solvent - water for and in 5 ml ampoules - 5 amps. - cardboard packs. 1 g - Glass bottles with a capacity of 10 ml (10) complete with solvent - water for and in ampoules 5 ml - 10 amps. - cardboard packs.
pharmachologic effect
Combined drug, broad-spectrum antibiotic.
Cefoperazone is a third-generation cephalosporin antibiotic that is bactericidal and has a wide spectrum of action; highly active against aerobic and anaerobic gram-positive and gram-negative microorganisms (including Pseudomonas aeruginosa), resistant to beta-lactamases of gram-positive and gram-negative microorganisms.
Sulbactam is an irreversible inhibitor of beta-lactamases, which are secreted by microorganisms resistant to beta-lactam antibiotics; prevents the destruction of penicillins and cephalosporins under the influence of beta-lactamases of resistant microorganisms; binding to penicillin-binding proteins, it exhibits synergism when used simultaneously with penicillins and cephalosporins.
The combination of cefoperazone + sulbactam is active against all microorganisms sensitive to cefoperazone and exhibits synergism (reduces the MIC of the combination by up to 4 times compared to the values for each component separately) against microorganisms: Haemophilus influenzae, Bacteroides spp., Staphylococcus spp., Acinetobacter calcoaceticus, Enterobacter aerogenes, Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, Morganella morganii, Citrobacter freundii, Enterobacter cloacae, Citrobacter diversus.
Active in vitro against gram-positive bacteria - Staphylococcus aureus (including strains that form and do not form penicillinase), Staphylococcus epidermidis, Streptococcus pneumoniae, Streptococcus pyogenes (beta-hemolytic strain of group A), Streptococcus agalactiae (beta-hemolytic strain of group B), most strains of beta-hemolytic Streptococcus spp., Enterococcus faecalis; gram-negative bacteria - Escherichia coli, Klebsiella spp., Enterobacter spp., Citrobacter spp., Haemophilus influenzae, Proteus mirabilis, Morganella morganii, Providencia rettgeri, Providencia spp., Serratia spp. (including Serratia marcescens), Salmonella spp., Shigella spp., Pseudomonas aeruginosa, Acinetobacter calcoaceticus, Neisseria gonorrhoeae, Neisseria meningitidis; Bordetella pertussis, Yersinia enterocolitica; anaerobic bacteria - Bacteroides fragilis, Fusobacterium spp., Peptococcus spp., Peptostreptococcus spp., Veillonella spp., Clostridium spp., Eubacter spp., Lactobacillus spp.
Pharmacodynamics and pharmacokinetics
The combination of sulbactam and cefoperazone affects bacteria sensitive to cefoperazone . It acts synergistically against Escherichia coli, Staphylococcus spp., Proteus mirabilis, Enterobacter aerogenes, Morganella morganii, Bacteroides spp., Enterobacter cloacae, Acinetobacter calcoaceticus, Klebsiella pneumoniae, Citrobacter freundii, Haemophilus influenzae, Citrobacter diversus.
Cefoperazone affects microorganisms by inhibiting the biosynthesis of bacterial cell wall mucopeptide
Sulbactam promotes the activity of cefoperazone against resistant microorganisms that produce beta-lactamases .
The degree of binding of cefoperazone plasma proteins is about 85%, sulbactam - 38%.
The half-life of cefoperazone is approximately 2 hours, sulbactam - 1 hour. Cefoperazone is excreted in the urine within 8 hours.
In case of liver dysfunction, serum and urinary elimination time are increased.
Pharmacokinetics
Cmax of sulbactam and cefoperazone after intravenous administration of the combination at a dose of 2 g (1 g of sulbactam and 1 g of cefoperazone) for 5 minutes averaged 130.2 μg/ml and 236.8 μg/ml, respectively. This reflects the higher Vd of sulbactam (18.0 to 27.6 L) compared to that of cefoperazone (10.2 to 11.3 L).
After intramuscular administration of 1.5 g of sulbactam/cefoperazone (500 mg of sulbactam, 1 g of cefoperazone), the Cmax of sulbactam and cefoperazone in serum was observed from 15 minutes to 2 hours after administration. Cmax in serum were 19.0 and 64.2 μg/ml of sulbactam and cefoperazone, respectively.
Both sulbactam and cefoperazone are well distributed in various tissues and body fluids, including bile, gallbladder, skin, appendix, fallopian tubes, ovaries, and uterus.
There is no data on the presence of any pharmacokinetic interaction between sulbactam and cefoperazone when administered as part of combination drugs.
With repeated use, no significant changes in the pharmacokinetic parameters of both components were noted. When the drug was administered every 8-12 hours, no accumulation was observed.
Approximately 84% of the sulbactam dose and 25% of the cefoperazone dose when administered in combination are excreted by the kidneys. The remaining part of cefoperazone is excreted mainly in bile. When the combination is administered, T1/2 of sulbactam averages about 1 hour, T1/2 of cefoperazone is 1.7 hours. Plasma concentration is proportional to the administered dose.
Cefoperazone is actively excreted in bile. T1/2 of cefoperazone is usually prolonged, and urinary excretion is increased in patients with liver disease and/or biliary tract obstruction. Even with severe liver dysfunction, a therapeutic concentration of cefoperazone is achieved in the bile, and T1/2 increases only 2-4 times.
In patients with varying degrees of renal impairment who received this combination, a high correlation was revealed between the total clearance of sulbactam from the body and the calculated QC. In patients with end-stage renal failure, a significant prolongation of T1/2 of sulbactam was detected (on average 6.9 hours and 9.7 hours in various studies). Hemodialysis caused significant changes in T1/2, total body clearance and Vd of sulbactam.
In elderly people with renal failure and impaired liver function, compared with healthy volunteers, an increase in T1/2 duration, a decrease in clearance and an increase in Vd of both sulbactam and cefoperazone were detected. The pharmacokinetics of sulbactam correlated with the degree of renal dysfunction, and the pharmacokinetics of cefoperazone correlated with the degree of liver dysfunction.
Studies in children did not reveal significant changes in the pharmacokinetic parameters of the components of the combination compared to adults. The average T1/2 of sulbactam in children ranged from 0.91 to 1.42 hours, cefoperazone - from 1.44 to 1.88 hours.
Indications of the active substances of the drug Cefoperazone + Sulbactam
Treatment of infectious and inflammatory diseases caused by microorganisms sensitive to the combination of cefoperazone + sulbactam: pharyngitis, tonsillitis, sinusitis, bronchitis, pneumonia, bronchopneumonia, empyema, lung abscess, pyelonephritis, cystitis, prostatitis, endometritis, gonorrhea, vulvovaginitis; peritonitis, cholecystitis, cholangitis; acute otitis media, sinusitis, tonsillitis; furunculosis, abscess, pyoderma, lymphadenitis, lymphangitis; osteomyelitis, joint infections, sepsis, meningitis.
Prevention of infectious complications after abdominal, gynecological and orthopedic operations, in cardiovascular surgery
Indications for use
The drug Cefoperazone + Sulbactam is used for urinary tract infections, respiratory tract infections, intraperitoneal infections, skin infections, osteomyelitis , sepsis , soft tissue infections, joint infections, ENT infections, meningitis .
The medicine is also prescribed for the prevention of infectious complications after orthopedic , abdominal , gynecological and cardiovascular surgeries .
Dosage regimen
The method of administration and dosage regimen of a particular drug depend on its release form and other factors.
The optimal dosage regimen is determined by the doctor. The compliance of the dosage form of a particular drug with the indications for use and dosage regimen should be strictly observed. Enter intramuscularly or intravenously.
The ratio of the components of the combination is 1:1.
For adults, the daily dose of the combination is 2-4 g (cefoperazone 1-2 g + sulbactam 1-2 g). The daily dose should be divided into equal parts and administered every 12 hours.
For severe or refractory infections, the daily dose of the combination can be increased to 8 g (cefoperazone 4 g + sulbactam 4 g).
For children, the daily dose of the combination is 40-80 mg/kg/day (cefoperazone 20-40 mg/kg + sulbactam 20-40 mg/kg). The daily dose should be divided into equal parts and administered every 6-12 hours.
For serious or treatment-resistant infections, the daily dose of the combination can be increased to 160 mg/kg.
In newborns, during the first week of life, the combination should be administered every 12 hours. The maximum daily dose of sulbactam in children should not exceed 80 mg/kg/day.
In patients with severe renal impairment (creatinine clearance 15-30 ml/min), the maximum dose of sulbactam is 1 g every 12 hours (the maximum daily dose of sulbactam is 2 g), and in patients with creatinine clearance less than 15 ml/min, the maximum dose of sulbactam is 500 mg every 12 hours (maximum daily dose of sulbactam - 1 g). For severe infections, additional administration of cefoperazone may be required. Since during hemodialysis the pharmacokinetics of sulbactam changes significantly and the serum T1/2 of cefoperazone is slightly reduced, administration of this combination should be planned after dialysis.
If regular monitoring of serum concentrations of cefoperazone is not carried out, then the minimum daily dose should not exceed 2 g.
If it is necessary to administer more than 80 mg/kg/day, calculated by the activity of cefoperazone, an increase in the dose is achieved through additional administration of cefoperazone.
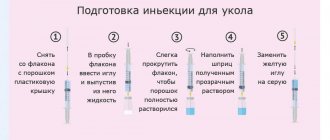
For intravenous bolus administration, the contents of the bottle are dissolved in an adequate volume of 5% dextrose solution, 0.9% sodium chloride solution, 5% dextrose solution in 0.225% sodium chloride solution, 5% dextrose solution in 0.9% sodium chloride solution or sterile water d/i , and injected over 3 minutes; for intravenous infusion, dissolve as stated above, dilute to 20-100 ml and administer over 15-60 minutes; for intramuscular administration, sterile water for dissolution is used.
Preparation of a solution using lidocaine: dilution is carried out in 2 stages - with sterile water, then with a 2% lidocaine solution to obtain a 0.5% lidocaine solution. The total volume of solvent is 6.7 ml.
Cefoperazone and Sulbactam Jodas 1 g + 1 g No. 1 bottle
Content
Pharmacodynamics Indications Contraindications Use with caution During pregnancy and lactation Method of administration and dosage Method of preparation Side effects Overdose Interaction Special instructions Effect on the ability to drive vehicles and machinery Storage conditions Shelf life
Pharmacodynamics
The antibacterial component of cefoperazone/sulbactam is cefoperazone, a third-generation cephalosporin that acts on sensitive microorganisms during their active reproduction by inhibiting the biosynthesis of cell wall mucopeptide.
Sulbactam sodium is a derivative of the main core of penicillin. Sulbactam does not have clinically significant antibacterial activity (except for Neisseriaceae and Acinetobacter). However, it has been noted that it is an irreversible inhibitor of most major beta-lactamases, which are produced by microorganisms resistant to beta-lactam antibiotics.
The ability of sulbactam to prevent the destruction of penicillins and cephalosporins by resistant microorganisms was confirmed in studies using resistant strains, in relation to which sulbactam had pronounced synergism with penicillins and cephalosporins. In addition, sulbactam interacts with some penicillin-binding proteins, so cefoperazone/sulbactam often has a more pronounced effect on susceptible strains than cefoperazone alone.
The combination of sulbactam and cefoperazone is active against all microorganisms sensitive to cefoperazone. In addition, it has synergism against various microorganisms, primarily: Haemophilus influenzae, Bacteroides spp., Staphylococcus spp., Acinetobacter calcociceticus, Enterobacter aerogenes, Escherichia coli. Proteus mirabilis, Klebsiella pneumoniae, Morganella morganii, Citrobacter freundii, Enterobacter cloacae, Citrobacter diversus.
Cefoperazone/sulbactam is active in vitro against a wide range of clinically significant microorganisms.
Gram-positive microorganisms:
Staphylococcus aureus (penicillinase-producing and non-penicillinase-producing), Staphylococcus epidermidis. Streptococcus pneumoniae, Streptococcus pyogenes (group A beta-hemolytic streptococcus), Streptococcus agalacliae (group B beta-hemolytic streptococcus), most other strains of beta-hemolytic streptococci. many strains of Streptococcus faecalis (enterococci).
Gram-negative microorganisms:
Escherichia coli. Klebsiella spp. (including Klebsiella pneumonia). Enterobacter spp. (including Enterobacter aerogenes and Enterobacter cloacae). Citrobacter spp. (including Citrobacter freundii and Citrobacter diversus), Haemophilus influenzae, Proteus mirabilis, Proteus vulgaris. Morganella morganii, Providencia rettgeri, Providencia spp., Serratia spp. (including Serratia marcescens), Salmonella and Shigella spp., Pseudomonas aeruginosa and some other Pseudomonas spp., Acinetobacter calcoaceticus, Neisseria gonorrhoeae, Neisseria meningitidis, Bordetella pertussis, Yersinia enterocolitica.
Anaerobic microorganisms:
- Gram-negative rods (including Bacteroides fragilis, other Bacteroides species and Fusobacterium spp.).
- Gram-positive and gram-negative cocci (including Peptococcus, Peptostreptococcus and Veillonella spp).
- Gram-positive rods (including Clostridium spp., Eubacterium spp. and Lactobacillus spp.).
The following sensitivity levels have been established for cefoperazone/sulbactam. The minimum inhibitory concentration (MIC) in mcg/ml expressed in the concentration of cefoperazone for sensitive microorganisms is less than or equal to 16, for organisms with intermediate sensitivity it is in the range of 17-63, and for resistant organisms it is more than 64. Sensitivity zones when determined by the disk diffusion method are: for sensitive microorganisms more than 21 mm; with intermediate sensitivity - from 16 to 20 mm, and for resistant - more than 15 mm.
To determine the MIC, the method of serial dilutions of sulbactam/cefoperazone in a 1:1 ratio in broth or agar media can be used.
To determine MIC by the disk diffusion method, it is recommended to use a disk containing 30 μg of sulbactam and 75 μg of cefoperazone.
The following quality control standards are recommended when using discs containing 30 mcg sulbactam and 75 mcg cefoperazone. For the control strain Acinetobacter spp. (ATCC 43498) zone diameter is 26-32; for Pseudomonas aeruginosa (ATCC 27853) – 22-28; for Escherichia coli (ATCC 25922) – 27-33; for Staphylococcus aureus (ATCC 25923) – 23-30.
Indications
Infectious and inflammatory diseases caused by microorganisms sensitive to cefoperazone + sulbactam:
- Infections of the upper and lower respiratory tract;
- Urinary tract infections;
- Peritonitis, cholecystitis, cholangitis and other intra-abdominal infections;
- Sepsis;
- Meningitis;
- Skin and soft tissue infections;
- Bone and joint infections;
- Gonorrhea;
- Inflammatory diseases of the pelvic organs, endometritis and other genital tract infections.
Contraindications
Hypersensitivity to sulbactam, cefoperazone or other cephalosporins, penicillin and beta-lactam antibiotics.
Carefully
- Severe renal and liver dysfunction;
- Newborns, including premature babies.
Use during pregnancy and breastfeeding
Sulbactam and cefoperazone penetrate the placental barrier.
Adequate clinical studies have not been conducted for use in pregnant women. Cefoperazone + Sulbactam is excreted in breast milk. During pregnancy and lactation, the drug is used only if the expected benefit to the mother outweighs the potential risk to the fetus and child.
If it is necessary to prescribe the drug during lactation, breastfeeding should be stopped.
Directions for use and doses
Intravenously and intramuscularly.
Use in adults:
In adults, sulbactam/cefoperazone is recommended for use in the following daily doses:
- Ratio 1:1.
- Sulbactam / Cefoperazone: 2.0 - 4.0.
- Sulbactam dose (g): 1.0 - 2.0.
- Cefoperazone dose (g): 1.0 - 2.0.
The daily dose should be divided into equal parts and administered every 12 hours.
For severe or refractory infections, the daily dose of cefoperazone/sulbactam can be increased to 8 g with a ratio of the main components of 1:1 (i.e. 4 g of cefoperazone).
Patients receiving cefoperazone/sulbactam in a 1:1 ratio may require additional administration of cefoperazone. The dose should be divided into equal parts and administered every 12 hours.
The recommended maximum daily dose of sulbactam is 4 g.
Use for renal impairment:
In patients with a creatinine clearance of 15-30 ml/min, the maximum dose of sulbactam is 1 g every 12 hours (the maximum daily dose of sulbactam is 2 g), and in patients with a creatinine clearance of less than 15 ml/min, the maximum dose of sulbactam is 500 mg every 12 hours ( the maximum daily dose of sulbactam is 1 g). For severe infections, additional administration of cefoperazone may be required.
The pharmacokinetics of sulbactam changes significantly during hemodialysis. The half-life of cefoperazone from blood serum is slightly reduced during hemodialysis. Therefore, administration of the drug should be planned after dialysis.
Use for liver dysfunction:
A dose change in case of impaired liver function may be required only in cases of severe obstruction of the biliary tract and severe liver disease, as well as in patients with a combination of hepatic and renal failure. In these cases, it is recommended to monitor the serum concentration of cefoperazone and adjust its dose if necessary. If regular monitoring of the serum concentration of cefoperazone is not possible, then its daily dose should not exceed 2 g.
Use in children:
In children, sulbactam/cefoperazone is recommended for use in the following daily doses:
- Ratio 1:1.
- Sulbactam / Cefoperazone (mg/kg/day): 40-80.
- Sulbactam dose (mg/kg/day): 20-40.
- Cefoperazone dose (mg/kg/day): 20-40.
The dose should be divided into equal parts and administered every 6-12 hours.
For serious or refractory infections, these dosages can be increased to 160 mg/kg/day for a 1:1 ratio of the main components. The daily dose is divided into 2-4 equal parts.
Use in newborns:
In newborns, during the first week of life, the drug should be administered every 12 hours. The maximum daily dose of sulbactam in children should not exceed 80 mg/kg/day.
Cooking method
Preparation of the solution.
- Total dose (g): 2.0.
- Equivalent doses of sulbactam + cefoperazone: 1.0 + 1.0.
- Solvent volume: 6.7.
- Maximum final concentration (mg/ml): 125 + 125.
Intramuscular administration:
Preparation of a solution using lidocaine. To prepare a solution for intramuscular administration, you can use a 2% solution of lidocaine hydrochloride, but it cannot be used for initial dissolution, given their incompatibility. Compatibility can be achieved by a two-step solution preparation - initially, the powder (2 g of Cefoperazone/sulbactam) is dissolved in 4.7 ml of sterile water for injection. Then the resulting solution is diluted with a 2% solution of lidocaine hydrochloride, adding 2 ml of local anesthetic to the solution obtained during the initial dilution. The total volume of solvent is 6.7 ml. The final solution will contain cefoperazone/sulbactam in a ratio of 125 mg/125 mg in 1 ml of 0.5% lidocaine solution. Injected deeply intramuscularly into areas of the body with an affected muscle layer (for example, the upper outer quadrant of the buttock).
Intravenous administration:
To prepare a solution for intravenous infusion, dilute 2 g (1 g + 1 g) of cefoperazone and sulbactam in an initial volume of 6.7 ml of one of the following and infusion solutions: 5% dextrose solution in water, 5% dextrose solution in 0.225% sodium chloride solution, 5 % dextrose solution in saline, 0.9% sodium chloride solution or sterile water for injection, and then diluted to 20 ml with the same solvent.
Preparation of a solution using Ringer's lactate. Since Ringer's lactate is not suitable for initial dilution, the solution is prepared in two stages: first, water for injection is used (see table above), and then the resulting solution is diluted with Ringer's lactate solution to a sulbactam concentration of 5 mg/ml (2 ml of the initial solution is diluted in 50 ml of lactated Ringer's solution or 4 ml in 100 ml of lactated Ringer's solution).
The infusion is carried out over 15-60 minutes.
For intravenous injection, the contents of each vial should be dissolved in 6.7 ml of one of the diluents described above and administered over a minimum of 3 minutes.
Side effects
- Blood and lymphatic system disorders: leukopenia, neutropenia, positive direct Coombs reaction, decreased hemoglobin, decreased hematocrit, thrombocytopenia, eosinophilia, hypoprothrombinemia.
- Immune system disorders: anaphylactoid reaction (including shock), hypersensitivity reaction (including anaphylactic shock).
- Nervous system disorders: headache.
- Vascular disorders: vasculitis, arterial hypotension.
- Gastrointestinal disorders: diarrhea, nausea, vomiting, pseudomembranous colitis.
- Disorders of the liver and biliary tract: increased activity of alanine aminotransferase, aspartate aminotransferase, blood alkaline phosphatase, increased concentration of bilirubin in the blood, jaundice.
- Skin and subcutaneous tissue disorders: pruritus, urticaria, toxic epidermal necrolysis, Stevens-Johnson syndrome, maculopapular rash.
- Renal and urinary tract disorders: hematuria.
- General disorders and disorders at the injection site: phlebitis at the infusion site, pain and burning at the injection site, fever, chills.
Overdose
Information on the acute toxicity of cefoperazone sodium and sulbactam sodium in humans is limited.
In case of overdose, you can expect undesirable effects recorded when using the drug.
It is necessary to take into account the fact that high concentrations of beta-lactam antibiotics in the cerebrospinal fluid can lead to neurological disorders, including seizures.
Treatment: symptomatic, hemodialysis is effective, especially in patients with impaired renal function.
Interaction
Solutions of cefoperazone/sulbactam and aminoglycosides should not be directly mixed, given the pharmaceutical incompatibility between them. If combination drug therapy is carried out, the two drugs are administered by sequential infusions using separate secondary catheters, and the primary catheter is sufficiently flushed with the solution between drug doses. Intervals Between injections during the day they should be as large as possible.
When consuming ethanol during treatment with cefoperazone and for up to 5 days after its administration, disulfiram-like effects may develop, characterized by hot flashes, sweating, headache and tachycardia. In patients who require artificial nutrition (orally or parenterally), the use of solutions containing ethanol should be avoided.
Compatible with water for injection, 5% dextrose solution, 0.9% sodium chloride solution, 5% dextrose solution in 0.225% sodium chloride solution, 5% dextrose solution in 0.9% sodium chloride solution. Incompatible with Ringer's solution, 2% lidocaine solution (initial use of water for injection results in a compatible mixture).
special instructions
Given the wide spectrum of activity of the drug, adequate monotherapy can be carried out.
The risk of hypersensitivity reactions, including those resulting in death, is higher in patients who have a history of hypersensitivity reactions to multiple allergens. If an allergic reaction occurs, it is necessary to discontinue the drug and prescribe adequate therapy.
Serious anaphylactic reactions require immediate administration of epinephrine. Oxygen is prescribed, corticosteroids are administered intravenously, and the airway is maintained, including intubation. Patients should be warned about the possibility of disulfiram-like effects when consuming alcoholic beverages during treatment with sulbactam and cefoperazone.
Dose changes may be required in cases of severe biliary obstruction, severe liver disease, and renal dysfunction associated with any of these conditions.
In patients with impaired liver function and concomitant impaired renal function, it is necessary to monitor the serum concentration of cefoperazone and adjust its dose if necessary. If regular monitoring of the serum concentration of cefoperazone is not carried out in such cases, then its daily dose should not exceed 2 g.
When using Benedict's or Fehling's solution, a false positive reaction to glucose in the urine may occur.
When using aminoglycosides concomitantly, it is necessary to monitor renal function.
Long-term use of the drug may disrupt the normal intestinal microflora, which can lead to the growth of Clostridium Difficile and cause the development of pseudomembranous colitis. In this case, it is necessary to discontinue the drug and prescribe specific treatment. The use of drugs that inhibit intestinal motility is contraindicated.
During treatment with cefoperazone, in rare cases, vitamin K deficiency developed. The reason for this is probably the suppression of the normal intestinal microflora, which synthesizes this vitamin. The risk group includes patients who receive poor nutrition, suffer from malabsorption (for example, with cystic fibrosis) and are on intravenous artificial nutrition for a long time. In such cases, as well as in patients receiving anticoagulants, it is necessary to monitor prothrombin time and, if indicated, prescribe vitamin K.
With prolonged treatment, excessive growth of insensitive microorganisms may occur. Patients must be carefully monitored during treatment. During long-term therapy, it is recommended to periodically monitor indicators of the function of internal organs, including the kidneys, liver and hematopoietic system. This is especially important for newborns, especially premature babies, and small children. The effectiveness and safety of use in newborns and children under 1 year of age has not been sufficiently studied. Before prescribing the drug, the potential benefits and possible risks should be weighed. Cefoperazone does not displace bilirubin from protein compounds in the blood plasma.
Impact on the ability to drive vehicles and machinery
Based on clinical experience with the use of cefoperazone + sulbactam, its effect on the ability to drive vehicles and operate machinery is unlikely.
Storage conditions
Store at a temperature not exceeding 30°C.
Prepared solutions of the drug for intravenous and intramuscular administration are stable for 24 hours at room temperature.
Keep out of the reach of children.
Best before date
3 years. Do not use after expiration date.
Side effect
Allergic reactions: anaphylactic shock.
From the digestive system: diarrhea, nausea, vomiting, pseudomembranous colitis; transient increase in liver function indicators - AST, ALT, alkaline phosphatase, bilirubin in the blood serum.
Allergic reactions: maculopapular rash, itching, urticaria, Stevens-Johnson syndrome (the risk of developing these reactions is higher in patients with a history of allergic reactions).
From the hematopoietic system: decreased number of neutrophils, reversible neutropenia (with long-term treatment), decreased hemoglobin and hematocrit levels, transient eosinophilia, leukopenia, thrombocytopenia, hypoprothrombinemia; in some cases - a positive Coombs test. When using Benedict's or Fehling's solution, a false-positive reaction to glucose in the urine may occur.
From the urinary system: hematuria.
Local reactions: sometimes after an intramuscular injection there is transient pain and a burning sensation at the injection site. When administered intravenously using a catheter, phlebitis may develop at the infusion site.
Other: headache, fever, chills, vasculitis.
Interaction
It is advisable not to combine with alcohol and products containing ethanol, including within 5 days after the course of the drug. Otherwise, negative reactions such as facial redness, headache , sweating , and tachycardia .
When using the drug Cefoperazone + Sulbactam, an erroneously positive reaction to glucose in the urine is possible if or Fehling's solution .
special instructions
When using aminoglycosides concomitantly, it is necessary to monitor renal function.
In patients with liver disease and/or biliary obstruction, T1/2 of cefoperazone increases and renal excretion is increased. In severe liver dysfunction, the concentration of cefoperazone in bile is therapeutic, T1/2 increases by 2-4 times. Changing the dose and monitoring the concentration of cefoperazone in the blood serum is required for severe obstruction of the biliary tract, severe liver failure (maximum daily dose - 2 g).
Patients who adhere to an inadequate diet or have malabsorption of food (patients with cystic fibrosis; patients on parenteral nutrition for a long time) are at risk of developing vitamin K deficiency. In such patients, prothrombin time should be monitored; if necessary, vitamin K is prescribed. The mechanism for the development of vitamin K deficiency is the suppression of the intestinal microflora, which normally synthesizes this vitamin.
With long-term treatment, it is necessary to monitor indicators of kidney, liver and hematopoietic system function.
During the treatment period, false-positive results of determining glucose in urine may be observed when using Benedict's or Fehling's solutions, or a false-positive Coombs reaction.
Treatment of premature newborns, pregnant women, and lactation is carried out if the possible benefit outweighs the potential risk.
Cefoperazone + Sulbactam por d/i/v and IM injection 500 mg + 500 mg vial No. 1
Contraindications
Cefoperazone/sulbactam is contraindicated in patients with hypersensitivity to penicillins, sulbactam, cefoperazone or any other cephalosporins.
Carefully:
Severe renal and liver dysfunction.
Newborns, including premature babies.
Directions for use and doses
Intravenous (IV) and intramuscular (IM).
Use in adults
In adults, cefoperazone + sulbactam is recommended for use in the following daily doses: The daily dose should be divided into equal parts and administered every 12 hours.
For severe or refractory infections, the daily dose of cefoperazone + sulbactam can be increased to 8000 mg with a ratio of the main components of 1:1 (i.e., 4000 mg of cefoperazone). Patients receiving cefoperazone + sulbactam in a 1:1 ratio may require additional administration of cefoperazone. The dose should be divided into equal parts and administered every 12 hours.
The recommended maximum daily dose of sulbactam is 4000 mg.
Use for renal impairment
In patients with a creatinine clearance of 15-30 ml/min, the maximum dose of sulbactam is 1000 mg every 12 hours (maximum daily dose of sulbactam 2000 mg), and in patients with a creatinine clearance of less than 15 ml/min, the maximum dose of sulbactam is 500 mg every 12 hours ( maximum daily dose of sulbactam 1000 mg).
For severe infections, additional administration of cefoperazone may be required.
The pharmacokinetics of sulbactam changes significantly during hemodialysis. Serum T1/2 of cefoperazone decreases slightly during hemodialysis. Therefore, administration of the drug should be planned after dialysis.
Use for liver dysfunction
Dose adjustments may be required in cases of severe biliary obstruction, severe liver disease, or in cases of renal dysfunction associated with any of these conditions.
In patients with impaired liver function and concomitant impaired renal function, it is necessary to monitor the serum concentration of cefoperazone and adjust its dose if necessary.
If the daily dose of cefoperazone does not exceed 2000 mg, there is no need to monitor its concentration in the blood serum (see section "Special Instructions").
Use in children
In children, cefoperazone + sulbactam is recommended for use in the following daily doses: The dose should be divided into equal parts and administered every 6-12 hours.
For severe or refractory infections, the daily dose can be increased to 160 mg/kg/day with a 1:1 ratio of the main components. The daily dose is divided into 2-4 equal parts.
Use in newborns
In newborns, during the first week of life, the drug should be administered every 12 hours. The maximum daily dose of sulbactam in children should not exceed 80 mg/kg/day.
Method for preparing solutions for parenteral use
Breeding:
Intramuscular administration:
Preparation of a solution using lidocaine. To prepare a solution for intramuscular administration, you can use a 2% solution of lidocaine hydrochloride, but it cannot be used for initial dissolution, given their incompatibility. Compatibility can be achieved by a two-step solution preparation - initially the powder is dissolved in sterile water for injection and then diluted with a 2% solution of lidocaine hydrochloride. The total volume of solvent is 6.7 ml. The final solution will contain cefoperazone/sulbactam in a ratio of 125 mg/125 mg in 1 ml of 0.5% lidocaine solution.
Intravenous administration:
To prepare a solution for infusion, dilute 2000 mg (1000 mg + 1000 mg) of the drug Cefoperazone + Sulbactam in an initial volume of 6.7 ml of one of the following infusion solutions: 5% dextrose solution, 5% dextrose solution in 0.225% sodium chloride solution, 5% dextrose solution in 0.9% sodium chloride solution, 0.9% sodium chloride solution or water for injection, and then diluted to 20 ml with the initial solution.
Preparation of a solution using Ringer's lactate
Since Ringer's lactate is not suitable for initial dilution, the solution is prepared in two stages: first, water for injection is used (see table above), and then the resulting solution is diluted with Ringer's lactate solution to a sulbactam concentration of 5 mg/ml (2 ml of the initial solution is diluted in 50 ml lactated Ringer's solution or 4 ml per 100 ml lactated Ringer's solution). The infusion is carried out over 15-60 minutes.
For intravenous injection, the contents of each vial should be dissolved in 6.7 ml of one of the solvents described in the preparation of solution for infusion (see above) and administered over a minimum of 3 minutes. The reconstituted solution is stored for 24 hours at room temperature.
Storage conditions
In a place protected from light, at a temperature not exceeding 25°C.
Keep out of the reach of children.
Best before date
3 years. Do not use after the expiration date.
special instructions
The development of hypersensitivity reactions, including those leading to death, has been reported during therapy with beta-lactam antibiotics, including cephalosporins, including cefoperazone + sulbactam.
The risk of hypersensitivity reactions, including those resulting in death, is higher in patients who have a history of hypersensitivity reactions to multiple allergens. If an allergic reaction occurs, it is necessary to discontinue the drug and prescribe adequate therapy.
In case of serious anaphylactic reactions (see section “Side Effects”), immediate administration of epinephrine and glucocorticosteroids is necessary; ensure airway patency, including intubation.
Severe skin reactions, such as toxic epidermal necrolysis, Stevens-Johnson syndrome and exfoliative dermatitis, in some cases fatal (see section "Side effects"), have been observed in patients receiving cefoperazone + sulbactam therapy. If severe skin reactions occur, use of cefoperazone + sulbactam should be discontinued and appropriate treatment should be initiated.
Patients should be warned about the possibility of disulfiram-like effects when consuming alcoholic beverages during treatment with Cefoperazone + Sulbactam.
Dose adjustments may be required in cases of severe biliary obstruction, severe liver disease, or in cases of renal dysfunction associated with any of these conditions.
In patients with impaired liver function and concomitant impaired renal function, it is necessary to monitor the concentration of cefoperazone in the blood serum and adjust its dose if necessary. If the daily dose of cefoperazone does not exceed 2000 mg, there is no need to monitor its concentration in the blood serum.
Cefoperazone does not displace bilirubin from protein compounds in the blood plasma.
Serious bleeding events, including death, have been observed in patients receiving cefoperazone + sulbactam therapy.
During treatment with cefoperazone, vitamin K deficiency developed, leading to coagulopathy. The reason for this is probably the suppression of normal intestinal microflora, which synthesizes this vitamin.
The risk group includes patients receiving malnutrition, with malabsorption syndrome (for example, with cystic fibrosis) and who have been on intravenous artificial nutrition for a long time. In such cases, as well as in patients receiving anticoagulants, it is necessary to monitor the prothrombin time and, if indicated, prescribe vitamin K. It is necessary to discontinue the use of cefoperazone + sulbactam if persistent bleeding occurs when there are no alternative causes for its manifestation.
With long-term treatment with Cefoperazone + Sulbactam, as with other antibiotics, excessive growth of insensitive microorganisms may occur. Patients must be carefully monitored during treatment. During long-term therapy, it is recommended to periodically monitor indicators of the function of internal organs, including the kidneys, liver, and systemic hematopoiesis. This is especially important for newborns, especially premature babies, and small children.
Cases of diarrhea associated with Clostridium difficile have been reported in association with the use of almost all antibacterial drugs, including cefoperazone + sulbactam. The severity of diarrhea can vary from mild to severe. Treatment with antibacterial drugs disrupts the normal intestinal microflora, which leads to excessive growth of Clostridium difficile. Clostridium difficile produces toxins A and B, which lead to diarrhea. Excessive amounts of toxins produced by Clostridium difficile strains may cause increased mortality in patients, as such infections may be resistant to antimicrobial therapy and may require colectomy. The use of drugs that inhibit intestinal motility is contraindicated. Possibility of developing diarrhea associated with Clostridium difficile. should be considered in all patients with diarrhea following antibiotic use. Close medical observation for 2 months is necessary for patients who experience diarrhea associated with Clostridium difficile after administration of antibacterial drugs.
Use in newborns
The drug Cefoperazone + Sulbactam is effective in young children. The use of this drug has not been studied extensively in newborns, including premature infants. Thus, before initiating therapy with Cefoperazone + Sulbactam in premature infants and newborns, the degree of benefit to the patient and the risk of developing serious adverse reactions should be assessed.
Description
Antibiotic, cephalosporin + beta-lactamase inhibitor.
Dosage form
Powder is white or white with a yellowish tint.
Reconstituted solution: clear, light yellow to yellow solution.
Use in children
With caution: newborns, including premature babies.
Pharmacodynamics
The antibacterial component of cefoperazone/sulbactam is cefoperazone, a third-generation cephalosporin that acts on sensitive microorganisms during their active reproduction by inhibiting the biosynthesis of cell wall mucopeptide.
Sulbactam does not have clinically significant antibacterial activity (except for Neisseriaceae and Acinetobacter). However, it has been noted that it is an irreversible inhibitor of most major beta-lactamases, which are produced by microorganisms resistant to beta-lactam antibiotics.
The ability of sulbactam to prevent the destruction of penicillins and cephalosporins by resistant microorganisms was confirmed in studies using resistant strains, in relation to which sulbactam had pronounced synergism with penicillins and cephalosporins. In addition, sulbactam interacts with some penicillin-binding proteins, so cefoperazone/sulbactam often has a more pronounced effect on susceptible strains than cefoperazone alone.
The combination of cefoperazone and sulbactam is active against all microorganisms sensitive to cefoperazone. In addition, it has synergism against various microorganisms, primarily: Haemophilus influenzae, Bacteroides spp., Staphylococcus spp., Acinetobacter calcoaceticus, Enterobacter aerogenes, Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, Morganella morganii, Citrobacter freundii, Enterobacter cloacae, Citrobacter diversus.
Cefoperazone/sulbactam is active in vitro against a wide range of clinically significant microorganisms.
Gram-positive microorganisms
Staphylococcus aureus (penicillinase and non-penicillinase producing), Staphylococcus epidermidis, Streptococcus pneumoniae, Streptococcus pyogenes (group A beta-hemolytic streptococcus), Streptococcus agalactiae (group B beta-hemolytic streptococcus), most other strains of beta-hemolytic streptococci, many strains of Streptococcus faecalis (enterococci).
Gram-negative microorganisms
Escherichia coli, Klebsiella spp., Enterobacter spp., Citrobacter spp., Haemophilus influenzae, Proteus mirabilis, Proteus vulgaris, Morganella morganii, Providencia rettgeri, Providencia spp., Serratia spp. (including Serratia marcescens), Salmonella spp. and Shigella spp., Pseudomonas aeruginosa and some other Pseudomonas spp., Acinetobacter calcoaceticus, Neisseria gonorrhoeae, Neisseria meningitidis, Bordetella pertussis, Yersinia enterocolitica.
Anaerobic microorganisms
Gram-negative rods (including Bacteroides fragilis, other Bacteroides spp. and Fusobacterium spp.).
Gram-positive and gram-negative cocci (including Peptococcus spp., Peptostreptococcus spp. and Veillonella spp).
Gram-positive rods (including Clostridium spp., Eubacterium spp. and Lactobacillus spp.).
The following sensitivity levels have been established for cefoperazone/sulbactam. The minimum inhibitory concentration (MIC) in mcg/ml, expressed in the concentration of cefoperazone, for sensitive microorganisms ≤16, for organisms with intermediate sensitivity is in the range of 17-63, and for resistant ones - more than 64. The sensitivity zones when determined by the disk diffusion method are : for sensitive microorganisms ≥21 mm; with intermediate sensitivity - from 16 to 20 mm, and for resistant ≤15 mm.
To determine the MIC, the method of serial dilutions of cefoperazone/sulbactam in a 1:1 ratio in broth or agar media can be used.
To determine MIC by the disk diffusion method, it is recommended to use a disk containing 30 μg of sulbactam and 75 μg of cefoperazone.
The following quality control standards are recommended when using discs containing 30 mcg sulbactam and 75 mcg cefoperazone. For the control strain Acinetobacter spp. (ATCC 43498) zone diameter is 26-32; for Pseudomonas aeruginosa (ATCC 27853) - 22-28; for Escherichia coli (ATCC 25922) - 27-33; for Staphylococcus aureus (ATCC 25923) - 23-30.
Side effects
In general, Cefoperazone + Sulbactam was well tolerated.
The severity of most adverse events was mild to moderate. The adverse reactions listed below were observed both during clinical studies (comparative and non-comparative) and after registration of the drug.
All adverse reactions listed in the instructions for medical use of the drug are presented in accordance with the MedDRA classification. In each frequency category, adverse reactions are presented according to the degree of clinical importance.
Frequency is presented according to CIOMS III (Council of International Organizations of Medical Sciences) categories: very common ≥ 1/10 (≥ 10%); often: ≥ 1/100 and < 1/10 (≥ 1% and < 10%); uncommon: ≥ 1/1000 and < 1/100 (≥ 0.1% and < 1%); frequency unknown: frequency cannot be determined from available data.
Blood and lymphatic system disorders
Very common: leukopenia§, neutropenia, positive direct Coombs reaction§, decreased hemoglobin§, decreased hematocrit§, thrombocytopenia§;
Common: coagulonation*, eosinophilia§;
Frequency unknown: hypoprothrombinemia*.
Immune system disorders
Not known: anaphylactic shock*†, anaphylactic reaction**, anaphylactoid reaction† (including shock)*, hypersensitivity reactions*†.
Nervous system disorders
Uncommon: headache.
Vascular disorders
Not known: bleeding*†, vasculitis*, hypotension*.
Gastrointestinal disorders
Common: diarrhea, nausea, vomiting;
Frequency unknown: pseudomembranous colitis*.
Disorders of the liver and biliary tract
Very often: increased activity of alanine aminotransferase (ALT) §, aspartate aminotransferase (AST) §, alkaline phosphatase in the blood§;
Often: increased concentration of bilirubin in the blood§;
Frequency unknown: jaundice*.
Skin and subcutaneous tissue disorders
Uncommon: itching, urticaria;
Not known: toxic epidermal necrolysis*†, Stevens-Johnson syndrome*†, exfoliative dermatitis*†, maculopapular rash*.
Renal and urinary tract disorders
Frequency unknown: hematuria*.
General disorders and disorders at the injection site
Uncommon: phlebitis at the infusion site, pain and burning at the injection site, fever, chills.
* Adverse events identified during post-registration use of the drug.
§ When calculating the frequencies of adverse reactions in the form of abnormal laboratory test results, all available data from the test results are taken into account, including data from patients in whom abnormalities were observed at baseline. This conservative approach was used because the baseline data did not allow us to distinguish between the subset of patients with abnormal laboratory results at baseline whose significant changes in laboratory results occurred after initiation of treatment and the subset of patients with abnormal laboratory results at baseline who experienced significant changes in laboratory results after initiation of treatment. there were no significant changes in laboratory test results.
For white blood cell counts, neutrophils, platelets, hemoglobin, and hematocrit, study reports report only abnormal values. It is not indicated whether there was an increase or decrease in indicators.
† Fatalities have been reported.
Use during pregnancy and breastfeeding
Proper clinical studies of the use of Cefoperazone + sulbactam in pregnant women have not been conducted.
Cefoperazone and sulbactam penetrate the placental barrier and into breast milk. During pregnancy and lactation, the drug is used only if the expected benefit to the mother outweighs the potential risk to the fetus and newborn.
Interaction
Solutions of the drug cefoperazone + sulbactam and aminoglycosides should not be mixed, given the pharmaceutical incompatibility between them.
If combination therapy with Cefoperazone + Sulbactam and an aminoglycoside is carried out, the two drugs are administered by sequential infusions using separate secondary catheters, and the primary catheter is washed sufficiently well between doses of the drugs. The intervals between the administration of Cefoperazone + Sulbactam and aminoglycoside during the day should be as long as possible.
Ethanol
Disulfiram-like effects characterized by flushing, sweating, headache and tachycardia have been reported when ethanol was taken during treatment with cefoperazone and for up to 5 days after its administration. In patients who require artificial nutrition (orally or parenterally), solutions containing ethanol should be avoided.
When using Benedict's or Fehling's solution, a false positive reaction to glucose in the urine may occur.
Overdose
Information on acute overdose of cefoperazone and sulbactam in humans is limited.
In case of overdose, you can expect the appearance of undesirable effects registered with the use of the drug. It is necessary to take into account the fact that high concentrations of beta-lactam antibiotics in the cerebrospinal fluid can lead to neurological disorders, including seizures. Treatment: symptomatic, hemodialysis is effective, especially in patients with impaired renal function.
Impact on the ability to drive vehicles and operate machinery
Based on clinical experience with use, the effect of the drug on the ability to drive vehicles and machines is unlikely.
Drug interactions
Compatible with water, 5% dextrose solution, 0.9% sodium chloride solution, 5% dextrose solution in 0.225% sodium chloride solution, 5% dextrose solution in 0.9% sodium chloride solution.
Incompatible with Ringer's solution, 2% lidocaine hydrochloride solution (initial use of water d/i leads to the formation of a compatible mixture); aminoglycosides (if combination therapy is necessary, it is carried out by sequential fractional intravenous infusion of two drugs, using 2 separate systems for intravenous transfusion; in the interval between doses, the system should be flushed with a compatible solvent).
Consumption of ethanol (at the same time or within the next 5 days after the end of treatment) increases the risk of developing a disulfiram-like reaction (“hot flashes”, increased sweating, headache, tachycardia).
Terms of sale
The drug is available with a prescription.