Despite the large arsenal of antihypertensive drugs, 20–30% of patients fail to control blood pressure (BP) even while taking a combination of 3–4 drugs from the main groups [1]. Given the existing differences in approaches to combination therapy of arterial hypertension (AH) in different clinical recommendations, combinations of renin-angiotensin system inhibitors with calcium channel blockers or thiazide diuretics are often considered the most rational [2–4]. Activation of the sympathetic nervous system (SNS) plays an important role in the pathogenesis of hypertension. Among the groups that can reduce the manifestations of activation of the SNS, β-blockers are first-line drugs. However, for a significant proportion of patients, the prescription of drugs in this group is impossible due to the presence of contraindications and adverse metabolic effects. Another possibility to suppress the activation of central sympathetic nuclei is the possibility of using centrally acting drugs. Currently, 2nd generation drugs are widely used, one of which is moxonidine (Physiotens, Abbott).
Features of the mechanism of action and pharmacokinetics of moxonidine
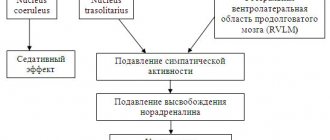
2nd generation centrally acting drugs are selective agonists of imidazoline receptors. These receptors are involved in the central regulation of SNS tone. Imidazoline receptors type 1 (I1) are localized in the central nervous system (CNS; in the nuclei of the reticular formation) and are involved in the negative feedback mechanism. Type 2 imidazoline receptors have been found in the periphery (for example, in the kidneys, pancreas, adipose tissue, heart). Activation of I1 receptors leads to a decrease in blood pressure and a decrease in heart rate (HR) [5]. Moxonidine is characterized by a pronounced antihypertensive effect, accompanied by a slight sedative effect. The moderate natriuretic effect is likely due to stimulation of renal I1 receptors and may contribute to the systemic hypotensive effect. Central I1 receptors of the hypothalamic region are also involved in the regulation of blood glycemia levels.
The physiological role of I1- and α-adrenergic receptors in the central nervous system has not been fully clarified. In experiments on animals under hypoxic conditions, it was shown that the activation of these receptors is associated with the functioning of the antioxidant defense system. The use of agonists of these receptors, clonidine and moxonidine, appears to be cerebroprotective and helps slow down the progression of vascular dementia [6]. Similarly, in an animal experiment it was shown that the use of moxonidine can reduce the risk of kidney damage during ischemia [7].
Infusion of moxonidine, even at a small dose, in spontaneously hypertensive rats led to an improvement in baroreceptor regulation of blood pressure. Infusion of a specific blocker of these receptors, yohimbine, suppressed baroreceptor sensitivity [8]. An experiment on rats showed that intravenous administration of moxonidine increases diuresis and natriuresis. Combined use with the α-adrenergic blocker prazosin reduced the diuretic effect of moxonidine, which suggested that activation of central I1 receptors leads to the activation of α1-adrenergic receptors, which mediate an increase in diuresis [9].
In atrial tissue, I1 receptors mediate an increase in the synthesis of natriuretic peptides. This was shown in an experiment on rats with isolated atrial circulation. Moxonidine increased atrial natriuretic peptide levels, but the central α-agonist clonidine did not. This may be one of the additional components of the antihypertensive effect of moxonidine [10].
Studies of the pharmacokinetics of moxonidine have shown that the drug is absorbed quite quickly and completely from the gastrointestinal tract (GIT). The maximum concentration of moxonidine in the blood is created 0.5–2.0 hours after administration. The main route of excretion is through the urine and only in small quantities through the gastrointestinal tract. Moxonidine in the body undergoes both oxidation and a phase II metabolic reaction (conjugation with cysteine) [11]. The main indication for the use of moxonidine is hypertension. For the treatment of hypertension, the drug is prescribed at a dose of 0.2–0.6 mg/day. With a single use, moxonidine allows you to control blood pressure throughout the day, which is confirmed by 24-hour monitoring data. The ratio of residual to peak effects when treated with this drug is 0.7 [12]. An animal study showed that stopping the administration of moxonidine did not lead to the development of the “rebound” phenomenon and an increase in blood pressure, in contrast to 1st generation centrally acting drugs (clonidine, etc.), which were characterized by “rebound” hypertension [13] .
The sympatholytic effects of moxonidine lead to changes in central hemodynamics. This was demonstrated in a study of a group of 22 patients with hypertension, left ventricular myocardial hypertrophy, a positive exercise test and the absence of significant coronary artery stenosis. 2 hours after taking 0.4 mg moxonidine, blood pressure decreased by 28/10 mmHg. Art., heart rate decreased by 5 beats/min. The pressure in the pulmonary circulation decreased - the average pressure in the pulmonary artery decreased by 17%, the wedge pressure of the pulmonary arteries - by 26%. The pressure in the left ventricle, its work and the need for oxygen decreased. Total coronary reserve increased by 12%. The concentration of norepinephrine in the blood decreased by 30%, in the endothelium - by 20% [14].
Antihypertensive efficacy of moxonidine: real practice and clinical trial data
In real clinical practice, moxonidine is most often used as a component of combination therapy. In one of the cohort studies, the frequency of prescription of 990 patients with hypertension and the main indications for treatment with moxonidine were analyzed. The drug was used by 4.5% of patients. Two thirds of patients received moxonidine due to hypertension resistant to conventional antihypertensive therapy. Another 33% of patients noted intolerance to first-line antihypertensive drugs. They received the drug in monotherapy [15].
In a group of 748 patients, an open study assessed the features of the use of moxonidine in the treatment of hypertension in combination with metabolic syndrome (MS): 81.1% of patients received it as part of combination therapy. The most common dose of moxonidine was 0.4 mg/day; 98% of patients completed 6 months of treatment. No serious side effects were observed when using the drug, blood pressure decreased significantly, and no adverse metabolic effects were recorded [16].
In an open study, it was shown that in patients with hypertension of the 1st and 2nd degrees, a dose of moxonidine 0.2 mg/day was effective in 62% of patients; in another 36% of patients, normalization of blood pressure was achieved when taking the drug at a dose of 0.4 mg/day . This study showed that the maximum reduction in blood pressure was achieved in the 3rd week from the start of therapy, and then the effect remained at the same level throughout the year of observation. An obvious advantage of the drug, therefore, turned out to be that tolerance to the drug does not develop even with long-term use [17].
Moxonidine has been studied in more than 70 studies in the treatment of hypertension. In total, more than 1,500 patients participated in phase II and III studies. However, the drug was well tolerated. Only 9% of patients noted dry mouth, 5–8% drowsiness, and 6% headaches. No more than 4% of patients refused to take medications due to side effects. Taking moxonidine did not worsen the course of diabetes mellitus and chronic obstructive pulmonary disease. In post-marketing studies, two cases of allergic reactions were noted. Thus, moxonidine had a good safety profile [18].
A number of studies have been conducted to compare the effectiveness of moxonidine with antihypertensive drugs of other groups. Moxonidine has been shown to have comparable antihypertensive activity to first-line drugs in the treatment of hypertension, angiotensin-converting enzyme inhibitors, β-blockers and diuretics [19–21].
Currently, moxonidine is a drug that is used primarily in combination therapy, but this drug has a number of organoprotective properties, as well as favorable metabolic effects, which allow us to formulate additional indications for its use.
Organoprotective properties of moxonidine
The effect on the state of the endothelium is considered one of the most important surrogate endpoints in assessing the effectiveness of antihypertensive therapy. The effect of moxonidine on endothelial function has been assessed in several small studies. In a group of 26 patients with obesity and hypertension, it was shown that moxonidine therapy in combination with a hypocaloric diet for 3 months leads to improved tissue sensitivity to insulin and improves the condition of the vascular endothelium compared to a group of patients receiving only a hypocaloric diet. During therapy with moxonidine, a significant increase in the degree of endothelium-dependent vasodilation in the reactive hyperemia test was observed compared with the control group [22]. In a small group of hypertensive patients with signs of insulin resistance (IR; HOMA index - Homeostasis Model Assessment > 2.77), the effectiveness of moxonidine and amlodipine was compared. The drugs had a comparable hypotensive effect. A more pronounced decrease in triglyceride levels was noted in the moxonidine group. The dynamics of the levels of uric acid, glucose, insulin, high and low density lipoproteins, biomarker YKL (tyrosine - Y, lysine - K, leucin - L)-40, which is a marker of endothelial dysfunction and the formation of atherosclerotic lesions of the vessel wall, did not differ significantly in patients receiving drugs from different groups [23].
In a group of 56 patients with essential hypertension and microalbuminuria, the effectiveness of moxonidine in monotherapy was studied. Patients received the drug at a dose of 0.3–0.4 mg/day for 6 months. During therapy with moxonidine, blood pressure significantly decreased and the severity of albuminuria decreased. In addition, a decrease in the concentration of thrombomodulin in blood plasma and a decrease in the level of plasminogen activator inhibitor were noted. The authors of the study associate the dynamics of the levels of these markers with the normalization of the endothelium during treatment [24].
A prospective randomized study compared the clinical and pharmacoeconomic effectiveness of treatment of patients with chronic kidney disease with moxonidine and nitrendipine. The study included patients with hypertension and non-terminal chronic renal failure. Over 3 years of observation, in the group of patients receiving nitrendipine, end-stage chronic renal failure developed in 38% of patients, in the moxonidine group - in 7%. The total cost of treatment with moxonidine in this group of patients was 4 times lower compared to treatment with nitrendipine [25].
Moxonidine may promote regression of left ventricular hypertrophy (LVH). This was shown when patients with hypertension were treated with moxonidine for 6 months, and the ejection fraction did not change significantly [19]. The reduction in LVH was also confirmed in experimental studies. The mechanism of action of moxonidine on the cardiac muscle may be associated with the activation of imidazoline receptors localized in the heart and the effect of the drug on the regulation of apoptosis and DNA stabilization [26].
The use of moxonidine may reduce the frequency and duration of paroxysms of atrial fibrillation. This was shown in a crossover design study in a group of 56 patients with paroxysmal atrial fibrillation. Patients were treated with moxonidine or placebo for 6 weeks. The effectiveness of therapy was monitored using 48-hour electrocardiogram monitoring. During treatment with moxonidine, a significant decrease in diastolic blood pressure and a decrease in the average duration of atrial fibrillation per day from 28 to 16 minutes were noted. The antiarrhythmic effect of moxonidine is associated with its sympatholytic activity [27].
Use of moxonidine in special groups of patients
MS is most often considered as an additional indication for prescribing moxonidine. The use of the drug promotes the activation of lipolysis of adipose tissue and weight loss. This was shown in the large open-label CAMUS study, where 4005 patients with hypertension in combination with obesity or MS received moxonidine as monotherapy and as part of combination therapy. During the 8 weeks of the study, during therapy with moxonidine, a decrease in the body weight of patients by an average of 1.4 kg was noted. In patients with obesity at the time of inclusion in the study, the decrease in body weight was more pronounced [28]. IR and hyperinsulinemia are the leading components of the pathogenesis of MS. The ability of moxonidine to influence tissue sensitivity to insulin has been studied in several studies. In a study involving 99 patients with hypertension and diabetes mellitus, the effectiveness of moxonidine in monotherapy and a combination of moxonidine and irbesartan was compared. Patients included in the treatment did not receive antihypertensive or glucose-lowering therapy. First, for 3 months, all patients received moxonidine at a dose of 0.2 mg, then some patients doubled this dose, and the second half of patients added irbesartan to therapy at a dose of 150 mg/day. Blood pressure, carbohydrate and lipid metabolism were monitored. Both treatment regimens caused a significant decrease in blood pressure, however, only with the use of moxoniline at a dose of 0.4 mg/day there was a decrease in the level of blood glucose and glycated hemoglobin. Improved insulin sensitivity index. A significant increase in high-density lipoprotein cholesterol levels was noted [29].
In a group of 77 obese patients with hypertension, the effect of moxonidine therapy on the insulin sensitivity index was studied. Patients received treatment with moxonidine or placebo for 8–9 weeks. It turned out that, in general, moxonidine caused a significant increase in the index of tissue sensitivity to insulin, especially pronounced in patients who had signs of IR [30].
It has been shown that moxonidine helps reduce blood glucose levels in patients with MS and improve tissue sensitivity to insulin, in contrast to 1st generation centrally acting drugs (alpha-methyldopa and clonidine) [31].
When comparing the effectiveness of treatment with moxondine at a dose of 0.2–0.6 mg/day and metoprolol at a dose of 50–150 mg/day, it turned out that the decrease in systolic and diastolic blood pressure during treatment with these drugs was the same. However, in the moxonidine group, by the end of therapy, fasting blood glucose levels were lower and an increase in the insulin sensitivity index was noted. In the metoprolol group, the insulin sensitivity index decreased, and fasting glucose levels were higher than during treatment with moxonidine [32].
A good tolerability profile and the absence of significant pharmacokinetic interactions create conditions for the use of moxonidine in the treatment of hypertension in elderly patients.
In a group of 14 elderly patients with hypertension resistant to treatment with three drugs, the effectiveness of moxonidine in combination therapy was studied. Blood pressure control was carried out according to clinical measurements and 24-hour blood pressure monitoring. Moxonidine was prescribed at a dose of 0.2–0.4 g/day. With the addition of this drug to treatment, according to clinical blood pressure monitoring, significant dynamics of both systolic (decrease from 195.9 ± 19.6 to 174 ± 17.8 mm Hg) and diastolic blood pressure (decrease from 103. 6 ± 9.5 to 99.0 ± 12.4 mmHg). According to 24-hour monitoring, daytime systolic blood pressure decreased by 15.4 mm Hg. Art., diastolic – at 7.4. At night, only systolic blood pressure significantly decreased (by 9.3 mm Hg). Thus, 11 out of 14 patients noted very good tolerability of moxonidine [33].
The sedative and sympatholytic effects of moxonidine allow us to recommend the drug for the treatment of hypertension in peri- and postmenopausal women. Moxonidine can help relieve menopausal symptoms in women. This was shown in a randomized trial of 112 postmenopausal women not receiving hormone replacement therapy, who were hypertensive and obese. Patients received atenolol or moxonidine. Both drugs significantly reduced blood pressure and helped relieve palpitations, hot flashes, shortness of breath, and insomnia. There were no differences between groups in the effect on clinical symptoms [34]. Only moxonidine increased tissue sensitivity to insulin. This pattern was observed in women with initial IR and was more pronounced among women who achieved good BP control during moxonidine therapy [35]. Moxonidine therapy contributed to a decrease in the area under the curve of the glucose tolerance test and a significant decrease in plasma insulin levels [36].
When comparing the effect of the same drugs on the level of inflammatory cytokines in postmenopausal women, it turned out that both atenolol and moxonidine help reduce the activity of tumor necrosis factor. However, treatment with atenolol also resulted in a decrease in adiponectin activity. Moxonidine did not significantly affect its level. Thus, the use of moxonidine appears to be preferable [37].
Another study in a group of 55 women with hypertension without diabetes mellitus showed that moxonidine therapy can significantly lower blood pressure and reduce the levels of glucose, cholesterol, triglycerides, albuminuria and IR index. At the same time, the level of adiponectin increased. A significant negative correlation was shown between the dynamics of the IR index and adiponectin [38].
Thus, moxonidine is a drug with proven antihypertensive effectiveness, a convenient dosage regimen and good tolerability. The drug can be recommended as one of the possible components of combination therapy for patients with resistant hypertension or in the presence of contraindications to the use of drugs of the main groups. Data on the effect of moxonidine on tissue sensitivity to insulin and the absence of adverse metabolic effects in the drug allow us to consider diabetes mellitus, obesity and MS as additional indications for prescribing this drug. Moxonidine can also be used to treat hypertension in elderly patients, as well as in postmenopausal women. The drug also has organoprotective properties - it reduces the severity of microalbuminuria, improves endothelial function, helps reduce LVH and prevent paroxysms of atrial fibrillation.
Centrally acting drug moxonidine in the treatment of arterial hypertension
According to epidemiological studies, the prevalence of arterial hypertension (AH) among adults in developed countries ranges from 20% to 40% and increases with age [3]. Elevated blood pressure is found in more than 50% of men and women over 60 years of age [4]. The relevance of the problem is supported by the increasing processes of urbanization of society, which create the preconditions for the emergence of risk factors such as stress, physical inactivity, obesity, bad habits and disturbed ecology. High blood pressure is one of the main risk factors for the development of cerebral stroke, coronary heart disease (CHD) and other cardiovascular diseases of atherosclerotic origin, which are associated with more than 50% of all deaths [5]. According to modern national recommendations [6], recommendations of the European Society of Arterial Hypertension and the European Society of Cardiology [7], the treatment tactics for essential hypertension depend on the level of blood pressure and the degree of risk of cardiovascular complications. The main goal of treatment is to minimize the risk of developing cardiovascular complications and death from them. The main objectives are normalization of blood pressure levels in order to prevent complications in the absence or minimal level of adverse drug reactions, correction of all modifiable risk factors (smoking, dyslipidemia, hyperglycemia, obesity), prevention, slowing down the rate of progression and/or reducing target organ damage, as well as treatment of associated and concomitant diseases - ischemic heart disease, diabetes mellitus (DM), etc. [6, 7]. When treating patients with hypertension, blood pressure should be less than 140/90 mm Hg. Art., which is its target level. If the prescribed therapy is well tolerated, it is advisable to reduce blood pressure to lower values. In patients with a high and very high risk of cardiovascular complications, it is necessary to reduce blood pressure to 140/90 mmHg. Art. or less within 4 weeks. In the future, subject to good tolerance, it is recommended to reduce blood pressure to 130/80 mmHg. Art. and less. In patients with coronary artery disease, blood pressure should be reduced to the target value of 130/85 mm Hg. Art. In patients with diabetes and/or kidney disease, the target blood pressure level should be less than 130/85 mmHg. Art. [6]. There are no uniform recommendations regarding which drugs should be used to start treating a patient. The choice of drug depends on many factors, including age, gender and the presence of concomitant diseases. Currently, 5 main classes of antihypertensive drugs are recommended for the treatment of hypertension: angiotensin-converting enzyme inhibitors (ACEIs), AT1 receptor blockers (ARBs), calcium antagonists (CAs), β-blockers (BABs), and diuretics. α-blockers, imidazoline I1 receptor agonists, and direct renin inhibitors can be used as additional classes for combination antihypertensive therapy [6, 7].
Agonists of imidazoline receptors (moxonidine, rilmenidine) are modern drugs with a central mechanism of action - they reduce the activity of the vasomotor center of the medulla oblongata. Centrally acting antihypertensive drugs also include clonidine, guanfacine, and methyldopa [1, 2]. Moxonidine (Moxarel, JSC "Vertex", Russia) is a highly selective agonist of imidazoline I1 receptors located in the rostral ventrolateral part of the medulla oblongata. Stimulation of these receptors reduces sympathetic flow and, accordingly, blood pressure by reducing vascular resistance. In addition, the activity of the vasomotor center decreases, cardiac output and peripheral sympathetic activity decrease. Taking moxonidine leads to a decrease in systemic vascular resistance and blood pressure. Moxonidine reduces the levels of renin, angiotensin II and aldosterone in the blood plasma [20]. After oral administration, the peak concentration is reached within 1 hour. The half-life in plasma is 2 hours and increases with renal failure. Despite the relatively short half-life, blood pressure is effectively regulated with a single dose of the drug per day. The antihypertensive effect lasts much longer, which is due to the retention of the drug in the central nervous system. The sympathoinhibitory effect of moxonidine is probably mediated almost entirely by its effect on I receptors. Moxonidine differs from other sympatholytic antihypertensive drugs in its lower affinity for central α2-adrenergic receptors, which explains the lower likelihood of developing sedation and dry mouth compared to clonidine. The antihypertensive effectiveness of moxonidine in essential hypertension has been proven in large-scale, double-blind, placebo-controlled, randomized studies and is comparable to that of most other antihypertensive drugs [8].
Food intake does not affect the pharmacokinetics of the drug. The drug is well absorbed from the gastrointestinal tract and is almost completely absorbed in its upper sections. Absolute bioavailability is approximately 88%. The maximum concentration in the blood is recorded after 0.5–3 hours. The connection with blood plasma proteins is 7.2%. 90% of the drug is excreted by the kidneys, mainly (70%) unchanged. The half-life (T1/2) of moxonidine and metabolite is 2.5 and 5 hours, respectively. Despite its short half-life (about 3 hours), it controls blood pressure throughout the day [9]. Moxonidine is effective in monotherapy for hypertension, but it is optimal to prescribe it as part of combination therapy, for example, with ACE inhibitors, sartans (ARBs), thiazide diuretics and other main antihypertensive drugs. The combination of moxonidine with other antihypertensive drugs enhances their antihypertensive effect. Moxonidine, when added to therapy with an ARB II (eprosartan), normalizes blood pressure and sympathetic hyperactivity in hypertensive normovolemic patients with chronic renal failure. In terms of antihypertensive effectiveness, moxonidine is not inferior to diuretics, beta blockers, AK and ACE inhibitors, and in terms of tolerability it is significantly superior to previous centrally acting drugs. It is safer than clonidine, propranolol, captopril, nifedipine. Moxonidine improves the insulin sensitivity index by 21% (compared to placebo) in patients with obesity, insulin resistance and moderate hypertension. The sedative effect is significantly less pronounced than that of other centrally acting drugs. The drug potentiates the effects of central nervous system depressants - ethanol, tranquilizers, barbiturates. Moxonidine may moderately improve impaired cognitive function in patients receiving lorazepam. Prescribing moxonidine together with benzodiazepines may be accompanied by an increase in the sedative effect of the latter [1, 6, 8–10, 13–19].
The TOPIC (Trial Of Physiotens In Combination) study, conducted in the UK at 138 clinical sites, included 566 patients with hypertension aged 18–80 years. When prescribing moxonidine at a dose of 0.2 or 0.4 mg/day, reliable control with monotherapy was achieved in 294 (52%) patients, in the rest - with combination therapy (in combination with amlodipine or enalapril). During the study, the drug proved to be effective and well tolerated both in monotherapy and combination therapy [10, 11]. When using moxonidine in patients with hypertension, a dual mechanism of action is observed - the drug provides both short-term (mainly due to its effect on the sympathetic centers of the brain) and long-term (by suppressing the release of renin and improving excretory function of the kidneys) blood pressure control. Thus, a single oral dose of moxonidine (0.4 mg) caused a statistically significant decrease in blood pressure in patients with hypertension from an average of 176/105 mm Hg. Art. up to 158/95 mm Hg. Art. [10, 11].
For the treatment of patients with mild and moderate hypertension, the recommended initial dose of Moxarel (moxonidine) is 0.2 mg/day. If the response to treatment is unsatisfactory, after 2 weeks. the dose is doubled. A dose of 0.2–0.4 mg/day in most cases was sufficient to maintain blood pressure at a satisfactory level. The high antihypertensive efficacy of moxonidine has been confirmed in the treatment of patients with uncomplicated hypertensive crisis. Thus, with sublingual administration of moxonidine at a dose of 0.4 mg, an effective reduction in blood pressure with good tolerability of the drug was achieved in 90% of patients. A significant decrease in systolic blood pressure and diastolic blood pressure after a single dose of the drug is observed after 20 minutes and reaches a maximum after 1.5 hours [1, 2, 10, 12]. Moxarel (moxonidine) occupies a special place in the treatment of hypertension combined with obesity. By activating imidazoline I1 receptors, it helps reduce fat hydrolysis, reduce free fatty acids, increase glucose metabolism and increase insulin sensitivity, reduce triglyceride levels, increase high-density lipoproteins and reduce plasminogen activator inhibitor-1 levels. Studies have provided evidence of the effect of moxonidine on reducing insulin resistance in overweight patients with impaired glucose tolerance. Thus, in the comparative study ALMAZ, the effect of moxonidine and metformin on glycemic control in patients with overweight, mild hypertension, insulin resistance and impaired glucose tolerance was assessed. The criteria for inclusion of patients in the study were: age over 40 years, body mass index (BMI) >27 kg/m2, fasting glucose >6.1 mmol/l. The ALMAZ study showed that moxonidine lowered fasting glucose levels, reduced patients' weight, increased the rate of glucose utilization, and reduced insulin resistance. With moxonidine, fasting glucose levels decreased less pronounced than with metformin, but insulin levels significantly decreased, while metformin had no effect on it, and BMI decreased equally with both drugs. Both drugs statistically significantly increase insulin sensitivity after a glucose load. Moxonidine affects the level of insulin in the blood, metformin regulates glucose levels, which is accompanied by a decrease in glycosylated hemoglobin. Both drugs statistically significantly reduce body weight while remaining metabolically neutral to lipids [21–26].
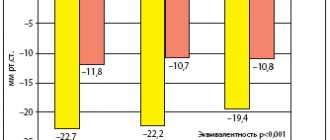
Long-term therapy with moxonidine in elderly patients with hypertension of 1-2 degrees provides an optimal reduction in blood pressure during the day and at night with good tolerability of the drug (low frequency and insignificant severity of side effects), leads to a significant reduction in left ventricular hypertrophy, and a decrease in the left ventricular myocardial mass index. Monotherapy with moxonidine for 24 weeks. has a positive effect on cognitive functions (memory and thinking), which indicates improved functioning of the frontal lobes of the brain. In the field of intellectual activity, positive dynamics were observed - the ability of patients to perform actions that require a higher level of generalization in the visual-figurative and visual-logical spheres increased. In addition, during the treatment, positive changes in memory were revealed - the productivity of memorization increased [27]. For patients with metabolic syndrome, it is also important to note the nephroprotective effect of moxonidine. Long-term use of moxonidine causes a significant decrease in microalbuminuria, concentrations of free thrombomodulin and plasminogen activator inhibitor in the blood. According to the results of a post-marketing review study, moxonidine effectively reduces blood pressure in patients with metabolic syndrome and at the same time promotes weight loss in obese patients. After 8 weeks treatment, patients recorded a decrease in body weight by an average of 1.4 kg, with the most pronounced decrease observed in obese patients. The frequency of use of combination antihypertensive therapy was 81.1% among patients with metabolic syndrome and 63.3% in the group as a whole. There was a significant decrease in systolic (26.9±15.1 mmHg, 95% CI 26.4–27.3) and diastolic (13.2±9.5 mmHg, 95% CI 12 ,9–13.5) BP. High efficacy of moxonidine (diastolic blood pressure reduction to <90 mm Hg and/or diastolic blood pressure reduction >10 mm Hg) was reported in 94% of patients. Similarly, a positive effect was observed in a large number of patients with metabolic syndrome - 94%, obesity - 93%, diabetes - 94% and those receiving monotherapy - 95% [28–31].
Conclusions. Moxarel (moxonidine) is the drug of choice among antihypertensive drugs with a central mechanism of action and is characterized by high antihypertensive efficacy and tolerability in patients with overweight, obesity, metabolic syndrome or without it, and also has additional metabolic properties and has a beneficial effect on body weight. Moxonidine is well tolerated, has little interaction with other drugs and can be used once a day in most patients. Moxarel (moxonidine) is indicated for the treatment of patients with mild to moderate hypertension, and especially as an additional drug in the treatment of patients with metabolic syndrome.
Literature 1. Clinical pharmacology and pharmacotherapy / Ed. V.G. Kukesa, A.K. Starodubtseva. M.: GEOTAR-Media, 2012. 832 p. 2. Clinical pharmacology: national guidelines (National Guidelines Series). M.: GEOTAR-Media, 2014. 976 p. 3. Oganov R.G., Timofeeva T.N., Koltunov I.E. and others. Epidemiology of arterial hypertension in Russia. Results of federal monitoring 2003–2010. // Cardiovascular therapy and prevention. 2011. No. 1. P. 9–13. 4. ABC Of Hypertension / Ed. by D. Gareth Beevers, Gregory H. Lip and Eoin O'Brien. 5th ed. Malden, Mass.: BMJ Books, 2007. P. 88. 5. Ezzati M., Lopez AD, Rodgers A. et al. Selected major risk factors and global and regional burden of disease // Lancet. 2002. Vol.360(9343). P.1347–1360. 6. Diagnosis and treatment of arterial hypertension. Recommendations of the Russian Medical Society on arterial hypertension and the All-Russian Scientific Society of Cardiologists. Fourth revision // Systemic hypertension. 2010. No. 3. P. 5–26. 7. ESH-ESC Guidelines Committee. 2013 guidelines for the management of arterial hypertension // J. Hypertens. 2013. Vol. 31. P. 1281–1357. 8. Neumann J., Ligtenberg G., Oey L. et al. Moxonidine normalizes sympathetic hyperactivity in patients with chronic renal failure receiving eprosartan // J. Amer. Soc. Nephrol. 2004. Vol.15. P. 2902–2907. 9. Instructions (TCFS) for the drug Moxarel. 10. Gaponova N.I., Abdrakhmanov V.R., Baratashvili V.L., Tereshchenko S.N. Analysis of the effectiveness and safety of moxonidine in patients with arterial hypertension and hypertensive crises // Practical Medicine. Cardiology. 2011. 04. 11. Waters J., Ashford J., Jager BA et al. Use of moxonidine as unitial therapy and in combination in the treatment of essential hypertension: results of the TOPIC (Trial of Physiotens in Combination) study // J. Clin Basic Cardiol. 1999. Vol. 2. P. 219–224. 12. Ruksin V.V., Grishin O.V. Emergency care for high blood pressure that is not life-threatening // Cardiology. 2011. No. 2. P. 45–51. 13. Frei M., Küster l., Gardosch von Krosigk PP. et al. Moxonidine and hydrochlorothiazide in combination: a synergistic antihypertensive effect // J. Cardiovasc. Pharmacol. 1994. Vol. 24. P. 25–28. 14. Prichard BNC, Simmons R, Rooks MJ et al. A double-blind comparison of moxonidine and atenolol in the management of patients with mild – to moderate hypertension // J. Cardiovasc. Pharmacol.1992. Vol. 20. P. 45–49. 15. Wolf R. The treatment of hypertensive patients with a calcium antagonist or moxonidine: a comparison // J. Cardiovasc. Pharmacol. 1992. Vol. 20. P. 42–44. 16. Lotti G., Gianrossi R. Moxonidin vs. captopril in mild to moderate hypertension (German) // Fortschr. Med. 1993. Vol. 111 (27). P. 429–432. 17. Kraft K., Vetter H. Twenty – four – hour blood pressure profiles in patients with mild – to – moderate hypertension; moxonidine versus captopril // J. Cardiovasc. Pharmacol. 1994. Vol. 24 (Suppl. 1). S. 29–S33. 18. Küppers HE, Jäger BA, Luszick JH et al. Placebo-controlled comparison of the efficacy and tolerability of once – daily moxonidine and enalapril in mild – to moderate essential hypertension // J. Hypertens. 1997. Vol.15. P. 93–97. 19. Prichard BNC, Kuster LJ, Hughes PR et al. Dose relation of blood pressure reduction with moxonidine: findings from three placebo – and active – controlled randomized studies // J. Clin. Basic. Cardiol. 2003. Vol. 6. P. 49–51. 20. Sanjuliani AE, Genelhu de Abreu V., Ueleres Braga J., Francischetti EA Effects of moxonidine on the sympathetic nervous system, blood pressure, plasma renin activity, plasma aldosterone, leptin and metabolic profile in obese hypertensive patients // J. Clin Basic Cardial. 2004. Vol. 7. P. 19–25. 21. Metabolic syndrome. M.: “MEDpres-inform”, 2007. 22. Mkrtumyan A.M., Biryukova E.V. The main approach to pharmacotherapy of metabolic syndrome // Consilium medicum. 2006. T. 8, No. 5. P.54–57. 23. Mychka V.B., Chazova I.E. Metabolic syndrome. Possibilities of diagnosis and treatment. (Prepared on the basis of recommendations of VNOK experts on the diagnosis and treatment of metabolic syndrome), 2008. P. 1–16. 24. Chazova I.E., Almazov V.A., Shlyakhto E.V. Moxonidine improves glycemic control in patients with arterial hypertension and overweight compared with metformin: the ALMAZ study // Diabetes, Obesity and Metabolism. 2006. Vol. 8. P. 456–465. 25. Scarpello JH, Howlett HC Metformin therapy and clinical uses // Diab. Vase. Dis. Res. 2008. Vol. 5. P. 157–167. 26. Shilov A.M., Avshalumov A.Sh., Sinitsina E.N., Eremina I.V. Correction of risk factors in patients with excess body weight combined with insulin resistance and arterial hypertension // Breast Cancer. 2011. T. 19, No. 2. P.1–7. 27. Martynov A.I., Ostroumova O.D., Korsakova N.K. and others. The effect of the drug moxonidine (physiotens) on the state of the cardiovascular system and brain in elderly patients with arterial hypertension // Russian Journal of Cardiology. 2002. No. 4. 28. Hausberg M., Tokmak F., Pavenstadt H. Et al. Effects of moxonidine on sympathetic nerve activity in patients with end-stage renal disease // J. Hypertens. 2010. Jul 14. 29. Krespi PG, Makris TK, Hatzizacharias AN et al. Moxonidine effect on microalbuminuria, thrombomodulin, and plasminogen activator inhibitor-1 levels in patients with essential hypertension // Cardiovasc. Drugs Ther. 1998.Vol.12(5). P. 463–467. 30. Aleksanyan L.A., Polosyants O.B. Moxonidine in the modern treatment of cardiovascular diseases // Breast Cancer. 2010. No. 18. 31. Sharma AM, Wagner T., Marsalek P. Moxonidine in the treatment of overweight and obese patients suffering from metabolic syndrome: results of a post-marketing survey study (CAMUS) // Reviews of Clinical Cardiology. 2007. No. 10.