Make an appointment by phone: +7 (343) 355-56-57
+7
- About the disease
- Cost of services
- Sign up
- About the disease
- Prices
- Sign up
Blood pressure (BP)
is one of the indicators of human health. Its increase is called arterial hypertension (AH). Hypertension is divided into hypertension (or true hypertension) and symptomatic hypertension (increased blood pressure due to a disease).
Normal blood pressure levels are 120/80 mm Hg. Art. Depending on the level, there are different degrees of hypertension. But the main criterion for the severity of hypertension is not so much high blood pressure as damage to target organs. They usually turn out to be those organs that have a well-developed vascular network and are well supplied with blood - the brain, retina, heart, kidneys, peripheral arteries of the lower extremities. That is why the degrees and stages of hypertension are distinguished, depending on the level of increase in blood pressure and damage to target organs, respectively.
But, regardless of the reason for the increase, hypertension always needs correction. It is necessary to distinguish between emergency, life-threatening increases in blood pressure and blood pressure that do not need urgent reduction.
An emergency, life-threatening increase in blood pressure accompanied by the following symptoms:
- intense headache, nausea, vomiting
- disturbance of consciousness
- visual impairment, hearing impairment, sudden onset
- shortness of breath, suffocation
- chest pain
- heart rhythm disturbances
- individually high blood pressure numbers recorded for the first time
- in older people. Since high blood pressure leads to the symptoms indicated in point 1
In the event of a life-threatening increase in blood pressure, you should immediately call an ambulance.
Composition and release form
The drug is sold in pharmacies in the form of small tablets for oral administration. Externally, they are white in color with a soluble thin shell. Packaged in blisters of 14 pieces and secondary cardboard packaging. One pack contains 1-2 cells of 14-28 tablets and includes instructions for use.
The active component of the drug is moxonidine. Its amount in 1 tablet is 200 mcg.
Additional substances: castor oil, cellulose, magnesium stearate, Tween 80, Klucel and Aerosil.
pharmachologic effect
The drug belongs to the group of antihypertensive drugs. It is a direct agonist of imidazoline receptors. When used once, it helps to simultaneously restore the level of diastolic and systolic pressure. At the same time, it does not change the activity of the heart and does not affect the heart rate.
The drug is highly digestible, regardless of the time of meal. The biological availability of the drug is at least 88%. The highest concentration of the drug in the red channel is observed after 30 minutes or a maximum of 3 hours.
Moxonidine
Suction
After oral administration, moxonidine is rapidly and almost completely absorbed from the upper gastrointestinal tract. Absolute bioavailability is approximately 88%, indicating no significant first-pass effect. The time to reach maximum concentration is about 1 hour. Food intake does not affect the pharmacokinetics of the drug.
Distribution
The binding to plasma proteins is 7.2%.
Metabolism
The main metabolite is dehydrogenated moxonidine. The pharmacodynamic activity of dehydrogenated moxonidine is about 10% compared to moxonidine.
Removal
The half-life (T1/2) of moxonidine and metabolites is 2.5 and 5 hours, respectively. Within 24 hours, over 90% of moxonidine is excreted by the kidneys (about 78% unchanged and 13% as dehydriromoxonidine, other metabolites in the urine do not exceed 8% of the dose taken). Less than 1% is excreted through the intestines.
Pharmacokinetics in patients with arterial hypertension
Compared with healthy volunteers, patients with arterial hypertension show no changes in the pharmacokinetics of moxonidine.
Pharmacokinetics in old age
Clinically insignificant changes in the pharmacokinetic parameters of moxonidine were noted in elderly patients, probably due to a decrease in the intensity of its metabolism and/or slightly higher bioavailability.
Pharmacokinetics in children
Moxonidine is not recommended for use in patients under 18 years of age, and therefore pharmacokinetic studies have not been conducted in this group.
Pharmacokinetics in renal failure
Moxonidine excretion is significantly correlated with creatinine clearance (CC). In patients with moderate renal failure (creatinine clearance in the range of 30-60 ml/min), steady-state plasma concentrations and final T1/2 are approximately 2 and 1.5 times higher than in individuals with normal renal function (creatinine clearance more than 90 ml/min). min).
In patients with severe renal failure (creatinine clearance less than 30 ml/min), steady-state plasma concentrations and final T1/2 are 3 times higher than in patients with normal renal function. The administration of multiple doses of moxonidine leads to predictable accumulation in the body of patients with moderate and severe renal failure.
In patients with end-stage renal failure (creatinine clearance less than 10 ml/min) on hemodialysis, steady-state plasma concentrations and final T1/2 are 6 and 4 times higher, respectively, than in patients with normal renal function.
In patients with moderate renal failure, the maximum concentration of moxonidine in the blood plasma is 1.5-2 times higher.
In patients with impaired renal function, the dosage should be adjusted individually.
Moxonidine is excreted to a small extent during hemodialysis.
Contraindications
It is prohibited to take the medicine for the following conditions:
- Individual intolerance to the active or auxiliary components.
- Acute form of bradycardia.
- Severe arrhythmia.
- Glaucoma.
- Mental disorders.
- Cardiac failure.
- Previous Quincke's edema.
- The period of bearing a child and breastfeeding.
- Children under 18 years of age.
- Disturbance of the liver and kidneys.
- Angina pectoris.
- Lactose deficiency or intolerance.
- Various pathologies of the circulatory system.
Side effects
As a result of treatment with Moxonidine, patients may experience the following adverse reactions:
- High fatigue, malaise.
- Migraine.
- Sleep disorder - insomnia or drowsiness.
- Dizziness, headache.
- Dyspeptic manifestations - dry mouth, discomfort in the epigastrium, nausea, vomiting, abnormal stool.
- Increased nervousness and irritability.
- Swelling of peripheral tissues.
- A sharp decrease in pulse and blood pressure.
- Allergic signs are skin rash and severe itching.
Analysis of efficacy and safety of moxonidine in patients with hypertension and hypertensive crises
Arterial hypertension (AH) is the most common disease in the Russian Federation.
At the same time, in our country there remains a steady trend of a steady increase in the number of emergency medical calls for hypertensive crises, which are the most serious complication of hypertension (1, 2, 3). Thus, in the Russian Federation as a whole, in recent years the number of calls from emergency medical teams for hypertensive crises (HC) has increased on average by 1.5 times, accounting for up to 20% of all reasons for calls (4.5%), and the total number of calls per year reaches 50 million (6.7). Thus, providing effective and, at the same time, safe emergency therapy to patients with hypertensive crises remains an urgent problem that requires constant analysis and improvement. In connection with the emergence of new antihypertensive drugs and the results of randomized multicenter studies, their introduction into practice will undoubtedly help improve the prognosis and quality of life of patients with hypertension.
Unfortunately, a number of antihypertensive drugs, while effectively lowering blood pressure, can have an unfavorable metabolic effect, thereby neutralizing the positive qualities of the drugs. Thus, beta-blockers, which successfully stop the hyperactivity of the sympathetic nervous system, can worsen carbohydrate metabolism, provoking hyperglycemia (up to the development of hyperglycemic coma in patients with diabetes mellitus (8). By provoking bronchospasm, beta-blockers can worsen the course of bronchial asthma and chronic obstructive diseases .
Due to the leading role of sympathetic hyperactivity in the development of arterial hypertension and hypertensive crises, new generation sympatholytics, such as moxonidine and rilmenidine, have recently been actively introduced into clinical practice.
Sympatholytics
Sympatholytics reduce blood pressure by exerting a selective effect on the vasomotor centers of the medulla oblongata, which provide regulation of sympathetic tone (11, 12, 13). This pharmacological group originates from methyldopa and clonidine, later moxonidine (physiotens) and rilmenidine were synthesized. Due to differences in the points of application and pharmacological effects, sympathomimetics are usually divided into drugs of the first generation or “old” and drugs of the second generation or “new”.
As can be seen from table. 1 and fig. 1, methyldopa affects exclusively alpha2-adrenergic receptors located on the neurons of the nuclei of the solitary tract of the medulla oblongata. Clonidine is a mixed agonist, since, along with alpha2-adrenergic receptors, it also has the ability to stimulate I1-imidazoline receptors located in the ventrolateral parts of the medulla oblongata (14).
Table 1.
Points of application of the effects of sympatholytics
| Medicine | Receptors in the medulla oblongata |
| Methyldopa | Alpha2 adrenergic receptors |
| Clonidine | Alpha2-adrenergic receptors + I1-imidazoline receptors |
| Moxonidine | I1-imidazoline receptors >>alpha2-adrenergic receptors |
| Rilmenidine | I1-imidazoline receptors > alpha2-adrenergic receptors |
Figure 1. Points of application and antihypertensive effect of sympatholytics
Second generation drugs are selective agonists of I1-imidazoline receptors. In contrast to the “old” sympathomimetics, the new drugs, especially moxonidine, have low affinity for alpha2-adrenergic receptors in the brainstem (15, 16). As a result, the risk of side effects such as sedation and dry mouth is significantly reduced.
The selectivity of moxonidine for I1-imidazoline receptors is approximately 70 times greater than its selectivity for alpha2-adrenergic receptors, while rilmenidine is less selective and therefore less effective (17). It is also known that the interaction of centrally acting drugs with alpha2-adrenergic receptors in brain areas is responsible for problems associated with the development of side effects (sedation, depression, dry mouth, etc.) characteristic of “old” sympathomimetics (18, 19, 20). At therapeutic doses, moxonidine has no side effects and causes a dose-dependent decrease in blood pressure without the development of withdrawal syndrome and with a slight change in heart rate (21). A decrease in plasma renin levels and an increase in natriuresis was also detected, which is probably mediated by stimulation of imidazoline receptors in the kidneys (22).
Moxonidine reduces plasma levels of renin, angiotensin II, and aldosterone (22). These effects are of particular importance, since a decrease in the activity of the renin-angiotensin-aldosterone system (RAAS) prevents the development and progression of cardiac and vascular remodeling (23, 24, 25, 26).
A decrease in the hyperactivity of the sympathetic nervous system during treatment with moxonidine is accompanied by an increase in tissue sensitivity to insulin, an improvement in carbohydrate and lipid metabolism, in particular, a decrease in the level of plasma glucose and serum leptin (27, 28). The positive effect of moxonidine on carbohydrate and lipid metabolism makes it the drug of choice for the treatment of hypertension in patients suffering from diabetes mellitus and metabolic syndrome.
According to numerous double-blind randomized studies, moxonidine was not inferior in antihypertensive effectiveness to diuretics, beta-blockers, calcium antagonists and ACE inhibitors, and in tolerability it was significantly superior to previous centrally acting drugs (29-35).
It is important to note the stability of the antihypertensive effect of moxonidine - the ratio of the residual reduction in blood pressure to the maximum reduction in blood pressure - this figure should be at least 50%. For moxonidine it is 70%.
When using moxonidine in patients with hypertension, a dual mechanism of action is observed - the drug provides both short-term (mainly due to its effect on the sympathetic centers of the brain) and long-term blood pressure control (due to suppression of renin release and improvement of renal excretory function). Thus, a single oral dose of moxonidine (0.4 mg) caused a statistically significant decrease in blood pressure in patients with hypertension from an average of 176/105 mm Hg. Art. up to 158/95 mm Hg. Art. (36). At the same time, the antihypertensive effect of the drug was accompanied by a decrease in the initially elevated total vascular resistance from 1695 to 1427 dynes. sec/cm5, while cardiac output did not change significantly (Fig. 2).
Figure 2. Effect of moxonidine on cardiac output and total vascular resistance (according to V. Mitrovic et al., 1991)
An interesting feature of moxonidine was also revealed: in patients with hypertension, a higher initial blood pressure level is associated with a stronger decrease in blood pressure. In this regard, the possibility of relieving hypertensive crises with moxonidine is considered a promising area of application in clinical practice. In addition, the effectiveness and safety of the use of moxonidine in hypertension and a number of concomitant diseases, such as chronic obstructive pulmonary disease (COPD), diabetes mellitus, metabolic syndrome, etc., is noted.
Pharmacokinetics of moxonidine
When taken orally, absorption of moxonidine from the gastrointestinal tract is 90% (37). The maximum concentration in blood plasma after a single dose of 0.2 mg of the drug is achieved after 60 minutes. (36, 37). Bioavailability - 88%. Food intake does not affect the pharmacokinetics of moxonidine. The half-life of moxonidine and metabolites is 2.5 and 5 hours, respectively. Within 24 hours, more than 90% of the drug is excreted by the kidneys (37). Moxonidine does not accumulate with long-term use.
Repeated administration of moxonidine does not lead to accumulation in the body of patients, including patients with moderate renal failure (39). In the later stages, in patients with end-stage renal failure (creatinine clearance less than 10 mg/min) on hemodialysis, plasma concentrations and final plasma half-life (T1/2) are 6 and 4 times higher, respectively. than in patients with normal renal function. In such patients, the dose of the drug should be selected individually. Encouraging results confirming the renoprotective properties of moxonidine were obtained by J. Radermacher et al. (40). In 601 patients undergoing renal allotransplantation, moxonidine therapy resulted in a 70% reduction in the risk of allograft failure.
Use of moxonidine in clinical practice
The TOPIC (Titial of Physiotens in Combination) study, conducted in the UK at 138 clinical sites (42), included 566 hypertensive patients aged 18–80 years. When prescribing moxonidine at a dose of 0.2 mg or 0.4 mg per day, reliable control with monotherapy was achieved in 294 patients (52%), in the remaining patients - with combination therapy (in combination with amlodipine or enalapril). During the study, the drug proved to be effective and well tolerated both in monotherapy and in combination therapy.
The high antihypertensive efficacy of moxonidine has been confirmed in the treatment of patients with uncomplicated hypertensive crisis. Thus, with sublingual administration of moxonidine at a dose of 0.4 mg, an effective reduction in blood pressure with good tolerability of the drug was achieved in 90% of patients (5). As can be seen in Fig. 3, a significant decrease in systolic blood pressure and diastolic blood pressure after a single dose of the drug is observed after 20 minutes and reaches a maximum after 1.5 hours.
Figure 3. Changes in SBP (a) and DBP (b) with sublingual application of 0.4 mg moxonidine: before treatment (0); after 20 and 30 minutes; 1.5 and 3 hours after administration (according to V.V. Ruksin and O.V. Grishin, 2011)
SBP—systolic blood pressure; DBP - diastolic blood pressure
It is important to note that the examined patients did not have rebound syndrome, and dynamic monitoring of blood pressure levels confirmed the stable and long-term nature of the antihypertensive effect of moxonidine.
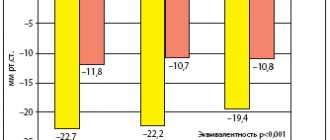
A positive effect of moxonidine on the course of hypertension was revealed in the treatment of patients who are very difficult to treat with antihypertensive therapy, namely, patients with COPD (43). As a rule, beta-blockers, as well as ACE inhibitors, which can provoke coughing and increased bronchial obstruction, are contraindicated in such patients. In a study of 40 patients with arterial hypertension due to COPD, treatment with moxonidine was accompanied by an improvement in the hemodynamics of the systemic and pulmonary circulation. Thus, the decrease in systolic blood pressure and diastolic blood pressure was 15.4 and 17.4%, respectively, reaching normal values. The pulse rate remained within normal limits during treatment and did not change significantly.
Thus, the selective inhibitor of I1-imidazoline receptors moxonidine (Physiotens) can be used as a universal antihypertensive drug, effective both for relieving hypertensive crises and for long-term treatment of arterial hypertension. Unlike “old” centrally acting drugs such as clonidine, methyldopa, moxonidine does not cause serious adverse events; There are also no hypotensive effects of the “first dose” and rebound syndrome. Moxonidine may be considered the drug of choice for the treatment of arterial hypertension and uncomplicated hypertensive crises in patients with diabetes mellitus, metabolic syndrome and chronic obstructive pulmonary diseases.
Since 2010, under the auspices of the VNOK section “Emergency Cardiology,” a randomized multicenter comparative study of the effectiveness of moxonidine in patients with uncomplicated hypertensive crisis has been conducted. The study is being conducted at 14 clinical centers in different regions of the Russian Federation. The study plans to include 280 patients with uncomplicated hypertensive crisis who are in the hospital at the time of inclusion in the study. Patients were randomized into two groups: group 1 (n=140) received a single oral dose of moxonidine at a dose of 400 mcg, control group 2 (n=140) received captopril at a dose of 25 mg. The duration of observation of the effect of moxonidine and captopril is 24 hours. The results of this study will be published this year.
N.I. Gaponova, V.R. Abdrakhmanov, V.L. Baratashvili, S.N. Tereshchenko
Literature:
1. Tereshchenko S.N. Hypertensive crises: diagnosis and treatment. In the book: Guide to arterial hypertension / ed. E.I. Chazova, I.E. Chazovoy. - Media Medica, 2005. - pp. 677-689.
2. Ruksin V.V. Emergency care for arterial hypertension: a brief guide for doctors. - M.: MEDpress-inform, 2009. - 48 p.
3. Gaponova N.I., Plavunov N.F., Tereshchenko S.N. and others. Clinical and statistical analysis of arterial hypertension complicated by hypertensive crisis in Moscow for 2005-2009. — Cardiology, 2011; 2: 40-44.
4. Golikov A.P. Crises in hypertension: yesterday and today. — Arterial hypertension, 2004; 3:23-27.
5. Ruksin V.V., Grishin O.V. Emergency care for non-life threatening high blood pressure. — Cardiology, 2011; 2: 45-51.
6. Healthcare in Russia 2005. - Stat. Collection. - M.: Rosstat, 2006. - 312 p.
7. Slepushenko I.A. Improving the organization of emergency medical care in the Russian Federation. — Ambulance, 2007; 3:3-6.
8. Diagnosis and treatment in cardiology / ed. M.H. Crawford. - Per. from English - M.: MEDpress-inform, 2007. - 800 p.
9. JNC VII - The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. US Department of Health and Human Services. National Institutes of Health. National Heart, Language and Blood Institute. National High Blood Pressure Education Program - NIH Publication No. 03-5233, 2003.
10. Recommendations for the prevention, diagnosis and treatment of arterial hypertension. - Arterial hypertension. - 2001. - 7 (Appendix), 1-16.
11. Wyss JM The role of the sympathetic nervous system in hypertension. Curr Opin Nephrol Hypertens, 1993; 2; 265-273.
12. Hamilton CA Chemistry, mode of action and experimental pharmacology of moxonidine. In: van Zwieten PA et al., editors. The I1-Imidazoline Receptor Agonist Moxonidine. 2nd Edition London. Roy Soc Med. 1996; 7-30.
13. Kobalava Zh.D., Kotovskaya Yu.V. Arterial hypertension 2000: key aspects of diagnosis, differential diagnosis, prevention, clinic and treatment. - M., 2001. - 208 p.
14. Van Zwieten PA, Thoolen MJ, Timmermans PB The hypotensive activity and side effects of methyldopa, clonidine and guanfacine. Hypertension, 1984; 6 (2): 28-33.
15. Ziegler D, Haxhiu MA, Kaan EC et al. Pharmacology of moxonidine, an I1-imidazoline receptor agonist. J. Cardiovasc. Pharmacol., 1996: 27 (suppl 3), S 26-S37.
16. Ernsberger PR, Westbrooks KL, Christen MO et al. A second generation of centrally acting antihypertensive agents act on putative I1-imidazoline receptors. J. Cardiovasc. Pharmacol. 1992; 20 (supll 4): S1-S10.
17. Schachter M., Mitchell G., Nizol C. et al. Antihypertensive efficacy of moxonidine in primary care: a “REAL-LIFE” study. Int J Clin Pract., 2003; 57 (6): 479-482.
18. Planitz V. Crossover comparison of moxonidine and clonidine HCL in mild to moderate hypertension. Eur J Clin Pharmacol 1984; 27: 147-152.
19. Planitz V. Comparison of moxonidine and clonidine in treating patients with hypertension. J Clin Pharmacol 1987; 27: 46-51.
20. Schwarz W., Kandziora J. Long-term experiences with moxonidine, a new antihypertensive. Fortsch. Med., 1990; 32: 616-620.
21. Prichard BNC., Küster LJ, Hughes PR et al. Dose relation of blood pressure reduction with moxonidine findings from three places—and active—comntrolled randomized studies. J. Clin Basic Cardiol. 2003; 6: 49-51.
22. Sanjuliani AE, Genelhu de Abreu V., Ueleres Braga J., Francischetti EA Effects of moxonidine on the sympathetic nervous system, blood pressure, plasma renin activity, plasma aldosterone, leptin and metabolic profile in obese hypertensive patients. J. Clin Basic Cardial., 2004; 7; 19-25.
23. Eichstädt H., Gatz G., Schröder R. et al. Left ventricular hypertrophy regression with moxonidine therapy. J Pharmacol Ther. 1991; 1:12-17.
24. Hüting J., Mitrovic V., Bahavar H. Comparison of the effects of moxonidine and nifedipine on left ventricular function during monotherapy of essential hypertension. Herz-Kreislauf. 1992; 24: 132-136.
25. Haczynski J., Spring A., Przewlocka-Kosmala M. et al. Effect of moxonidine on left ventricular hypertrophy in hypertensive patients. J. Clin Basic Cardiol., 2001; 4: 61-65.
26. Chazova I., Almazov VA, Shlyakhto E. Moxonidine improves glycaemic control in mildly hypertensive, overweight patients: a comparison with metformin. Diabetes, Obesity and Metabolism, 8, 2006, 456-465.
27. Julius S., Valentini M. Consequences of the increased autonomic nervous drive in hypertension, heart failure and diabetes. Blood Press, 1998; 7 (3): 5-13.
28. Huggett RJ, Scott EM, Gilbey SG et al. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation, 2003; 108:3097-3101.
29. Frei M., Küster l., Gardosch von Krosigk PP., et al. Moxonidine and hydrochlorothiazide in combination: a synergistic antihypertensive effect. J Cardiovasc Pharmacol 1994; 24: 25-28.
30. Prichard BNC, Simmons R, Rooks MJ, et al. A double-blind comparison of moxonidine and atenolol in the management of patients with mild to moderate hypertension. J Cardiovasc Pharmacol 1992; 20: 45-49.
31. Wolf R. The treatment of hypertensive patients with a calcium antagonist or moxonidine: a comparison. J Cardiovasc Pharmacol 1992; 20: 42-44.
32. Lotti G., Gianrossi R. Moxonidin vs. captopril in mild to moderate hypertension (German). Fortschr Med 1993; 111 (27); 429-432.
33. Kraft K, Vetter H. Twenty - four - hour blood pressure profiles in patients with mild - to - moderate hypertension; moxonidine versus captopril. J Cardiovasc Pharmacol 1994; 24 (suppl 1), S 29-S33.
34. Küppers HE, Jäger BA, Luszick JH et al. Placebo-controlled comparison of the efficacy and tolerability of once - daily moxonidine and enalapril in mild - to moderate essential hypertension. J. Hypertens 1997; 15: 93-97.
35. Prichard BNC, Kuster LJ, Hughes PR et al. Dose relation of blood pressure reduction with moxonidine: findings from three places—and active—controlled randomized studies. J Clin Basic Cardiol 2003; 6: 49-51.
36. Mitrovic V., Patyna W., Hütting J. et al. Hemodinamic and neurohumoral effecte of moxonidine in patients with essential hypertension. Cardiovase Drugs Ther. 1991; 5: 967-972.
37. Theodor R., Weimann H. J., Weber W. et al. Absolute bio-availability of moxonidine. Eur. J Drug Metab Pharmacokinet 1991; 16 (2): 153-159.
38. Weimann HJ, Rudolph M. Clinical pharmacokinetics of moxonidine, J. Cardiovasc. Pharmacol., 1992; 20 (suppl 4), S537-S541.
39. Kirch W., Hutt H., J., Plänitz V. The influence of renal function on clinical pharmacokinetics of moxonidine. Clin Pharmacokinet 1988; 15: 245-253.
40. Radermacher J., Mengel M., Ellis S. et al. The renal arterial resistance index and renal allograft survival. New Engl. J. Med., 2003; 349: 115-124.
41. Vonend O., Marsalex P., Russ H. et al. Moxonidine treatment of hypertensive patients with advanced renal failure. J.Hypertens. 2003; 21: 1709-1707.
42. Waters J, Ashford J, Jager BA et al. Use of moxonidine as unitial therapy and in combination in the treatment of essential hypertension: results of the TOPIC (Trial of Physiotens in Combination) study. J. Clin Basic Cardiol., 1999; 2: 219-224.
43. Adasheva T.V., Zadionchenko V.S., Matsievich M.V. and others. Arterial hypertension and COPD - a rational choice of therapy. RMJ, 2006; 10: 795-800.
Drug overdose
If medical recommendations are not followed and the maximum volume of the drug is exceeded, an overdose may develop. The condition is determined by severe symptoms:
- Intense headache and dizziness.
- Nausea followed by vomiting.
- Painful sensations in the stomach area.
- Weakness and severe malaise.
- Extensive reduction in pressure.
- Dryness in the mouth.
If the above symptoms occur, you should definitely consult a doctor. Treatment involves relief of symptoms. Prescribed medication, gastric lavage, parenteral administration of saline. To suppress the symptoms of bradycardia, the use of Atropine is indicated.
special instructions
The use of an antihypertensive drug for therapeutic purposes requires constant monitoring of blood pressure, heart function and heart rate.
During the course of treatment, you should completely avoid alcoholic beverages. It is recommended not to drive vehicles or engage in activities that require concentration, memory and high mental activity.
You need to stop taking the drug gradually, gradually reducing the dose until complete withdrawal.
If the use of Moxonidine was combined with beta-blockers and it is necessary to discontinue both drugs, the latter are removed first. After some time, the antihypertensive drug is discontinued.
How can you safely lower your blood pressure in an emergency?
- pull yourself together and calm down
- lie down and take 30-40 drops of Corvalol or Valoserdin in warm water
- take moxonidine (Physiotens) 0.2 mg under the tongue, repeat if blood pressure does not decrease within 15-20 minutes
If the patient is not taking moxonidine for the first time and the blood pressure numbers are significant (over 170/100 mmHg), then the patient can immediately take 0.4 mg of moxonidine sublingually. The maximum daily dose of moxonidine is 0.6 mg.
- call an ambulance and correctly formulate complaints and describe the situation: for example, we call an ambulance for an elderly patient with blood pressure readings of 220/120 mmHg and sudden onset of shortness of breath/chest pain/arrhythmia/loss of consciousness.
Correctly formulated complaints and a precisely defined situation will allow the EMS dispatcher to quickly navigate and send a specialized EMS team to the address. This will save time and save the patient's life.