Torasemide, 20 pcs., 5 mg, tablets
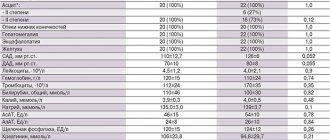
The incidence of side effects is classified according to the recommendations of the World Health Organization:
very common: ≥ 1/10 (> 10%);
often: from ≥ 1/100 to < 1/10 (> 1% and < 10%);
uncommon: >1/1000 to <1/100 (>0.1% and <1%);
rare: from ≥ 1/10000 to <1/1000 (> 0.01% and < 0.1%);
very rare: <1/10000 (<0.01%);
frequency unknown: frequency cannot be estimated from available data.
From the nervous system:
often - headache, dizziness, drowsiness;
infrequently - muscle cramps of the lower extremities;
frequency unknown - confusion, fainting, paresthesia in the extremities (feeling of numbness, “crawling” and tingling).
From the senses:
frequency unknown - visual impairment, hearing impairment, tinnitus and hearing loss (usually reversible), usually in patients with renal failure or hypoproteinemia (nephrotic syndrome).
From the cardiovascular system:
infrequently - extrasystole, arrhythmia, tachycardia;
frequency unknown - excessive decrease in blood pressure, orthostatic hypotension, collapse, deep vein thrombosis, thromboembolism, decrease in circulating blood volume.
From the respiratory system:
infrequently - nosebleeds.
From the digestive system:
often - diarrhea;
uncommon - abdominal pain, flatulence, polydipsia;
frequency unknown - dry mouth, nausea, vomiting, loss of appetite, pancreatitis, dyspeptic disorders, intrahepatic cholestasis.
For the skin and subcutaneous tissues:
frequency unknown - skin itching, rash, urticaria, erythema multiforme, exfoliative dermatitis, purpura, vasculitis, photosensitivity.
From the musculoskeletal system:
frequency unknown - muscle weakness.
From the urinary system:
often - increased frequency of urination, polyuria, nocturia;
infrequently - frequent urge to urinate;
frequency unknown - oliguria, urinary retention (in patients with urinary tract obstruction), interstitial nephritis, hematuria.
From the reproductive system:
frequency unknown - decreased potency.
From the side of metabolism:
frequency unknown - hypokalemia, hyponatremia, hypomagnesemia, hypocalcemia, hypochloremia, metabolic alkalosis, hypovolemia, dehydration (more often in elderly patients).
From the laboratory parameters:
uncommon - hypercholesterolemia, hypertriglyceridemia;
frequency unknown - hyperuricemia, a slight increase in the activity of alkaline phosphatase in the blood plasma, an increase in the concentration of creatinine and urea in the blood plasma, an increase in the activity of some “liver” enzymes in the blood plasma (for example, gamma-glutamyltransferase), thrombocytopenia, leukopenia, agranulocytosis, hyperglycemia, decrease glucose tolerance (possible manifestation of latent diabetes mellitus).
Other:
frequency unknown - aplastic or hemolytic anemia.
Torasemid-SZ
WHO classification of the incidence of side effects:
very often - >1/10 prescriptions (>10%)
often - from >1/100 to 1% and
infrequently - from >1/1000 to 0.1% and
rarely - from >1/10000 to 0.01% and
very rarely -
frequency unknown—cannot be estimated from available data
Blood and lymphatic system disorders: frequency unknown: thrombocytopenia, leukopenia, agranulocytosis, aplastic or hemolytic anemia.
Metabolic and nutritional disorders: uncommon: polydipsia, hypercholesterolemia, hypertriglyceridemia; frequency unknown: decreased glucose tolerance (possible manifestation of latent diabetes mellitus).
Nervous system disorders: often: dizziness, headache, drowsiness; uncommon: muscle cramps of the lower extremities; frequency unknown: confusion, fainting, paresthesia in the extremities (feeling of numbness, crawling and tingling).
Visual disorders: frequency unknown: visual impairment.
Hearing and labyrinthine disorders: frequency unknown: hearing impairment, tinnitus and hearing loss (usually reversible) usually in patients with renal failure or hypoproteinemia (nephrotic syndrome).
Cardiac disorders: uncommon: extrasystole, arrhythmia, tachycardia.
Vascular disorders: frequency unknown: excessive decrease in blood pressure, orthostatic hypotension, collapse, deep vein thrombosis, thromboembolism.
Respiratory, thoracic and mediastinal disorders: uncommon: nosebleeds.
Gastrointestinal disorders: often: diarrhea; uncommon: abdominal pain, flatulence; frequency unknown: dry mouth, nausea, vomiting, loss of appetite, pancreatitis, dyspeptic disorders.
Liver and biliary tract disorders: frequency unknown: intrahepatic cholestasis.
Skin and subcutaneous tissue disorders: frequency unknown: pruritus, rash, urticaria, erythema multiforme, exfoliative dermatitis, purpura, vasculitis, photosensitivity.
Musculoskeletal and connective tissue disorders: frequency unknown: muscle weakness.
Renal and urinary tract disorders: often: increased frequency of urination, polyuria, nocturia; uncommon: increased urge to urinate; frequency unknown: oliguria, urinary retention (in patients with urinary tract obstruction), interstitial nephritis, hematuria.
Disorders of the genital organs and breast: frequency unknown: decreased potency.
General disorders and administration site disorders: uncommon: fever, asthenia, weakness, fatigue, hyperactivity, nervousness. Severe anaphylactic reactions up to shock, which until now have only been described after intravenous administration.
Laboratory and instrumental data: frequency unknown: hypercholesterolemia, hypertriglyceridemia, hyperuricemia, a slight increase in the concentration of alkaline phosphatase in the blood, a transient increase in the concentration of creatinine and urea in the blood, an increase in the activity of some “liver” enzymes (for example, gamma-glutamyl transferase).
Disorders of water-electrolyte and acid-base balance: frequency unknown: hypokalemia, hyponatremia, hypomagnesemia, hypocalcemia, hypochloremia, metabolic alkalosis, hypovolemia, dehydration (more often in elderly patients), which can lead to hemoconcentration with a tendency to develop blood clots.
In case of an overdose of the drug, the following symptoms may develop: excessively increased diuresis, accompanied by a decrease in circulating blood volume (CBV) and an imbalance in the electrolyte balance of the blood, followed by a pronounced decrease in blood pressure, drowsiness and confusion, collapse. Gastrointestinal disturbances may occur.
A specific antidote is unknown. Vomiting is induced, gastric lavage is performed, and activated charcoal is prescribed. Treatment is symptomatic, dose reduction or discontinuation of the drug and at the same time replenishment of blood volume and indicators of water-electrolyte balance and acid-base status under the control of serum concentrations of electrolytes, hematocrit. Hemodialysis is not effective, since the elimination of torasemide and its metabolites is not accelerated.
THORASEMIDE
Side effects
The frequency of the side effects listed below was determined according to the following (World Health Organization classification): very common (more than 10%);
often (more than 1% and less than 10%); uncommon (more than 0.1% and less than 1%); rare (more than 0.01% and less than 0.1%); very rarely (less than 0.01%), including isolated reports; frequency unknown (cannot be estimated from available data). From the blood and lymphatic system
Frequency unknown
- thrombocytopenia, leukopenia, agranulocytosis, aplastic or hemolytic anemia.
Metabolism and nutrition
Infrequently
- hypercholesterolemia, hypertriglyceridemia.
Frequency unknown
- decreased glucose tolerance (possible manifestation of latent diabetes mellitus).
From the nervous system
Often -
dizziness, headache, drowsiness;
infrequently -
muscle cramps of the lower extremities;
frequency is unknown
- confusion, fainting, paresthesia in the extremities (feeling of numbness, “crawling” and tingling).
From the side of the organ of vision
Frequency unknown -
visual impairment.
Hearing and labyrinth disorders
Frequency not known? 1a —
Hearing impairment, tinnitus and hearing loss (usually reversible) usually occur in patients with renal failure or hypoproteinemia (nephrotic syndrome).
From the cardiovascular system
Infrequently -
extrasystole, tachycardia, increased heartbeat, facial flushing;
frequency not known -
excessive arterial hypotension, orthostatic hypotension, collapse, deep vein thrombosis, thromboembolism, hypovolemia.
From the respiratory system
Infrequently -
nosebleeds.
From the digestive system
Often
- diarrhea;
uncommon
- abdominal pain, flatulence, polydipsia;
frequency is unknown -
dry mouth, nausea, vomiting, loss of appetite, pancreatitis, dyspeptic symptoms, intrahepatic cholestasis.
From the kidneys and urinary tract
Often -
increased frequency of urination, polyuria, nocturia;
infrequently -
frequent urge to urinate;
frequency unknown
- oliguria, urinary retention (in patients with urinary tract obstruction), interstitial nephritis, hematuria, increased concentrations of urea and creatinine in the blood.
From the genital organs
Frequency unknown
- violation of potency.
General and administration site disorders
Infrequently
- asthenia (exhaustion), thirst, weakness, increased fatigue, hyperactivity, nervousness.
Laboratory indicators
Infrequently -
increase in platelet count;
frequency unknown -
hyperglycemia, hyperuricemia, decrease in the number of red blood cells, leukocytes and platelets, a slight increase in the activity of alkaline phosphatase in the blood, increased activity of some liver enzymes (for example, gamma-glutamyl transferase).
From the skin and subcutaneous tissues
Frequency unknown
- skin itching, rash, urticaria, erythema multiforme, exfoliative dermatitis, purpura, vasculitis, photosensitivity.
From the musculoskeletal system
Frequency unknown
- muscle weakness.
From the side of water-electrolyte and acid-base balance
Frequency unknown
- hyponatremia, hypochloremia, hypokalemia, hypomagnesemia, hypocalcemia, metabolic alkalosis. Symptoms indicating the development of electrolyte and acid-base disorders may include headache, confusion, convulsions, tetany, muscle weakness, cardiac arrhythmias and dyspeptic disorders; hypovolemia and dehydration (more often in elderly patients), which can lead to hemoconcentration with a tendency to develop thrombosis.
If any of the side effects indicated in the instructions get worse ,
or you notice any other side effects not listed in the instructions, tell your doctor.
Torasemide
special instructions
With caution: arterial hypotension; hypovolemia (with or without arterial hypotension); disturbances in the outflow of urine (benign prostatic hyperplasia, narrowing of the urethra or hydronephrosis); history of ventricular arrhythmia; acute myocardial infarction (increased risk of developing cardiogenic shock); diarrhea; pancreatitis; diabetes mellitus (decreased glucose tolerance); liver diseases complicated by cirrhosis and ascites, renal failure, hepatorenal syndrome; gout, hyperuricemia; anemia; simultaneous use of cardiac glycosides, amnoglycosides or cephalosporins, corticosteroids or ACTH; hypokalemia; hyponatremia; lactation period.
Patients with hypersensitivity to sulfonamides and sulfonylureas may have cross-sensitivity to torsemide.
In patients, especially at the beginning of treatment with torasemide and in the elderly, it is recommended to monitor the electrolyte balance, volume and concentration of circulating blood.
During long-term treatment with torasemide, it is recommended to regularly monitor electrolyte balance (especially potassium levels), glucose, uric acid, creatinine, lipids and cellular blood components.
In patients receiving torasemide in high doses, in order to avoid the development of hyponatremia and metabolic alkalosis, it is not advisable to limit salt intake.
The risk of hypokalemia is greatest in patients with liver cirrhosis, severe diuresis, insufficient dietary electrolytes, and concomitant treatment with corticosteroids or ACTH.
An increased risk of developing fluid and electrolyte imbalances is observed in patients with renal failure. During the course of treatment, it is necessary to periodically monitor the concentration of blood plasma electrolytes (including sodium, calcium, potassium, magnesium), acid-base status, residual nitrogen, creatinine, uric acid and, if necessary, carry out appropriate corrective therapy (with a higher frequency in patients with frequent vomiting and against the background of parenterally administered fluids).
In patients who have developed fluid and electrolyte disturbances, hypovolemia, or prerenal azotemia, laboratory findings may include: hyper- or hyponatremia, hyper- or hypochloremia, hyper- or hypokalemia, acid-base imbalance, and increased blood urea levels. If these disorders occur, it is necessary to stop taking torasemide until normal values are restored, and then resume treatment with torasemide at a lower dose. If azotemia and oliguria appear or worsen in patients with severe progressive kidney disease, it is recommended to suspend treatment.
The selection of a dosage regimen in patients with ascites against the background of liver cirrhosis should be carried out in a hospital setting (imbalances in water and electrolyte balance can lead to the development of hepatic coma). This category of patients requires regular monitoring of blood plasma electrolytes.
To prevent hypokalemia, it is recommended to use potassium supplements and potassium-sparing diuretics (primarily spironolactone), as well as follow a diet rich in potassium.
The use of torasemide may cause an exacerbation of gout.
In patients with diabetes mellitus or with reduced glucose tolerance, periodic monitoring of glucose concentrations in the blood and urine is required.
In patients with prostatic hyperplasia and narrowing of the ureters, diuresis control is necessary due to the possibility of acute urinary retention.
In patients with diseases of the cardiovascular system, especially those taking cardiac glycosides, diuretic-induced hypokalemia can cause the development of arrhythmias.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, patients should avoid engaging in potentially hazardous activities that require increased attention and speed of psychomotor reactions.