Khairabesol
Khairabesol
(
Hirabezol
) - a drug to reduce gastric acidity, a proton pump inhibitor, and an antiulcer.
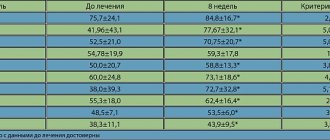
Treatment of erosive and ulcerative defects of the stomach and duodenum with the drug Khairabezol is clinically effective and has a persistent antisecretory effect. During a study assessing the effectiveness of Hairabezol, the following was noted. A decrease in pain in the epigastric region is observed from the 2nd day of use of the drug, and by the 7th day of therapy, the pain syndrome, according to the study, disappears. In the vast majority of patients, healing of erosive and ulcerative defects was achieved by the end of the 2nd week. The percentage of daily time pH>4 in the stomach increased by more than 2.5 times, while the percentage of time pH<4 in the esophagus decreased on average by almost 3 times. The total number of refluxes per day decreased on average by almost 4 times (V.A. Akhmedov, O.V. Gaus, etc.).
general information
According to the pharmacological index, Khairabezol belongs to the group “Proton pump inhibitors”.
According to ATC, it belongs to the group “Proton pump inhibitors” and has the code “A02BC04 Rabeprazole”. Proton pump inhibitors (in particular, rabeprazole) are considered the most effective drugs for controlling symptoms and treating complications of GERD, such as reflux esophagitis and Barrett's esophagus (see Fifth Moscow Agreement // XIII Congress of the National Registry, March 12, 2013).
Manufacturer: Highglans Laboratories, India. Dispensed from pharmacies with a doctor's prescription.
Dosage forms and composition of Khairabezol
The active substance of Khairabezol
is
rabeprazole
.
Khairabezol is available in the form of enteric film-coated tablets. The tablets are round, biconvex.
Tablets containing the active ingredient rabeprazole sodium 10 mg
covered with a shell of light to reddish-pink color.
Tablets containing rabeprazole sodium 20 mg
covered with a light yellow or yellow shell.
The tablets contain excipients magnesium oxide, mannitol, corn starch, povidone K30, low-substituted hyprolose, sodium stearyl fumarate.
The enteric-soluble coating contains cellacephate, titanium dioxide and red iron oxide dye (for Hayrabezol 10 mg tablets) or yellow iron oxide dye (for Khairabezol 20 mg tablets).
Indications for use of Khairabezol
From the instructions for the drug Khairabezol it follows that it is prescribed:
- for the treatment of duodenal and gastric ulcers in the acute stage, as well as anastomotic ulcers;
- for the treatment of reflux esophagitis;
- for the treatment of various forms of gastroesophageal reflux disease (GERD): non-erosive, erosive and ulcerative;
- for maintenance therapy of GERD;
- for the treatment of Zollinger-Ellison syndrome and other conditions characterized by pathological hypersecretion;
- for the combined treatment of peptic ulcer disease associated
with
Helicobacter pylori
.
Directions for use and dosage of Khairabezol
Khairabezol tablets are taken orally whole, without chewing or crushing. The timing of taking Khairabezol and simultaneous food intake do not affect the effectiveness of the drug.
- For gastric ulcers in the acute stage and anastomotic ulcers, it is recommended to take 10 mg or 20 mg of Hairabezol per day once. The standard course of treatment is 6 weeks, but in some cases it can be extended to 12 weeks.
- For duodenal ulcers in the acute stage, it is recommended to take 20 mg of Khairabezol once a day for 2-4 weeks. The course of treatment can be extended to 8 weeks if necessary. In some cases, the therapeutic effect occurs with a daily dose of 10 mg of the drug.
- For the treatment of erosive GERD or reflux esophagitis, a 4-8 week course of treatment with Khairabezol 10 mg or 20 mg once a day is recommended. Treatment can be extended for another 8 weeks if necessary. For maintenance therapy of GERD, Khairabezol is taken in the same dosage, the duration of treatment is determined by the patient's condition.
- For non-erosive GERD (NERD) without esophagitis, Hairabezol should be taken 10 mg or 20 mg once a day. If symptoms do not disappear after 4 weeks of treatment, additional examination of the patient is necessary. After eliminating the symptoms, in order to avoid their recurrence, a single dose of 10 mg of Hairabezol on demand is recommended.
- Treatment of Zollinger-Ellison syndrome and other conditions characterized by pathological hypersecretion with Khairabezol involves individual selection of the dosage of the drug. Starting from 60 mg per day, the daily dose is gradually increased to 100 mg in a single dose or 120 mg divided into 2 doses. For some patients, split doses of the drug are preferable. Treatment should be continued as clinically necessary. In cases of Zollinger-Elison syndrome, treatment can last up to one year.
- Combined treatment of duodenal ulcers and chronic gastritis associated with Helicobacter pylori
involves taking 20 mg of Hairabezol and 500 mg of clarithromycin with 1 mg of amoxicillin or 400 mg of metronidazole twice a day for a week. The use of double doses of Khairabezol increases the effectiveness of three-component therapy (see Fifth Moscow Agreement // XIII Congress of the NOGR. March 12, 2013).
Use of Khairabezol for functional dyspepsia
The choice of Hairabezol as maintenance therapy in patients with functional dyspepsia is justified by the following facts (Butorova L.I. et al.):
- Carrying out a three-month course of maintenance therapy with Khairabezol in a daily dose of 10 or 20 mg in patients with functional dyspepsia significantly reduces the recurrence rate and severity of dyspepsia symptoms compared with the results of using prokinetics and antacids
- the use of Hairabezol at a dose of 20 mg daily allows maintaining clinical remission of the disease in 80% of patients with FD, and at a dose of 10 mg - in 70%
- rapid relief of dyspepsia symptoms when using 20 mg of Khairabezol gives grounds in some cases to recommend maintenance treatment with this drug in an “on demand” mode
- a three-month course of maintenance therapy with Khairabezol was found to be well tolerated; side effects are observed in approximately 3% of patients and are most often expressed in a mild headache that does not require discontinuation of the drug or the use of additional medications
- Khairabezol is safe for long-term use and can be used in conjunction with other medications, including antacids
- Khairabezol has different dosages in tablet form (10, 20 mg) and differs from similar products in lower cost
Restrictions on use and contraindications
Khairabezole should be administered with caution to patients with severe hepatic impairment, since the AUC of rabeprazole in them is approximately 2 times higher than in healthy people.
Elderly patients and patients with renal insufficiency do not require dose adjustment.
Khairabezole should not be prescribed to children and adolescents under 18 years of age, pregnant and lactating women, since there are no sufficient studies to confirm the safety of rabeprazole in these categories of patients.
The FDA Fetal Risk Category for rabeprazole in pregnant women is C (animal studies have shown adverse effects of rabeprazole on the fetus and there have been no adequate studies in pregnant women, but the potential benefits associated with the use of this drug in pregnant women may justify its use despite at the existing risk).
Other medicines containing the active ingredient rabeprazole
In Russia, the following drugs with the active ingredient rabeprazole have been registered for sale in pharmacies: Bereta, Zolispan, Zulbex, Noflux, Ontime, Pariet, Rabelock, Rabeprazole-OBL, Rabeprazole-SZ, Rabiet, Razo. Most of them are available in the form of tablets for oral administration, coated with an intestinal soluble coating, containing 10 or 20 mg of the active substance - rabeprazole.
Side effects of Khairabezol
Side effects when taking Khairabezol most often occur from the digestive system (diarrhea, nausea) and from the nervous system (headache).
Materials for healthcare professionals regarding the use of Hairabezol
Articles
- Akhmedov V.A., Gaus O.V., Kiselev I.E., Kravtsova A.P. Evaluation of the effect of rabeprazole (drug "Hairabezol") 20 mg on 24-hour gastric pH-metry in patients with Hp-negative erosive and ulcerative lesions of the stomach and duodenum // Gastroenterology of St. Petersburg. 2015. No. 1–2. pp. 2–5.
- Akhmedov V.A., Gaus O.V. and others. Evaluation of the effectiveness of the drug hairabezol for the treatment of patients with erosive and ulcerative lesions of the stomach and duodenum // XX Ros. Gastroenterology Week October 6-8. RZHGGK. 2014. No. 5. P. 15.
- Orlov I.N. Diagnostic capabilities of 24-hour gastric pH metry in assessing the clinical effectiveness of the drug “hairabezol” in patients with negative erosive and ulcerative lesions of the stomach and duodenum // Abstracts of the VI International Youth Medical Congress “St. Petersburg Scientific Readings - 2015”. P. 50.
- Butorova L.I., Osadchuk M.A., Osadchuk M.M., Tokmulina G.M., Plavnik T.E., Smirnova N.A.. Optimization of therapeutic tactics for functional dyspepsia syndrome // Doctor.Ru. Gastroenterology. 2015. No. 12 (113). pp. 80-86.
- Spivakovsky Yu.M., Chernenkov Yu.V., Spivakovskaya A.Yu. and others. PPIs in the treatment of chronic gastroduodenitis in children. Materials XIX Ross. congr. "Innovative technologies in pediatrics and pediatric surgery." Russian Bulletin of Perinatology and Pediatrics.2020,65(4)223-4.
On the website GastroScan.ru in the “Literature” section there is a subsection “Rabeprazole”, containing articles devoted to the treatment of diseases of the gastrointestinal tract with rabeprazole preparations.
Video
Still from video by Partsvania-Vinogradova E.V. The role of pH-metry in acid-dependent diseases.
Still from video by E.S. Vyuchnova Tactics for managing patients with duodenogastric/biliary reflux
On the website GastroScan.ru in the “Video” section there is a subsection “For doctors”, containing video recordings of reports, lectures, webinars in various areas of gastroenterology for healthcare professionals, as well as a subsection “For students of medical universities”
Khairabezol has contraindications and side effects. Before use, you should consult a specialist. Back to section
Khairabezol, 15 pcs., 20 mg, enteric-coated tablets
Absorption
Rabeprazole is rapidly absorbed from the intestine and peak plasma concentrations (Cmax) are achieved approximately 3.5 hours after a 20 mg dose. Changes in peak plasma concentrations and area under the pharmacokinetic concentration-time curve (AUC) of rabeprazole are linear in the dose range from 10 to 40 mg. Absolute bioavailability after oral administration of 20 mg (compared to intravenous administration) is approximately 52%. In addition, bioavailability does not change with repeated dosing of rabeprazole. Neither the time of taking the drug during the day nor antacids affect the absorption of rabeprazole. Taking the drug with fatty foods slows down the absorption of rabeprazole by 4 hours or more, but neither Cmax nor the degree of absorption changes.
Distribution
In humans, the degree of binding of rabeprazole to plasma proteins is about 97%. Metabolism
Rabeprazole is metabolized in the body in two ways. A significant part of it is metabolized systemically by a non-enzymatic route with the formation of thioester derivatives. Rabeprazole is also metabolized in the liver via cytochrome P450 to form sulfonic and desmethyl derivatives.
In healthy volunteers, the plasma half-life is approximately 1 hour (varies from 0.7 to 1.5 hours), and the total clearance is 3.8 ml/min/kg.
Removal
After a single oral dose of 20 mg of 14C-labeled rabeprazole, about 90% of the drug is excreted in the urine, mainly in the form of carboxylic acid thioester, its glucuronide and in the form of mercapturic acid derivatives. No unchanged drug is detected in the urine. The remainder of the rabeprazole taken is excreted through the intestines. The total elimination is 99.8%.
End stage renal failure
In patients with stable end-stage renal disease who require maintenance hemodialysis (creatinine clearance <5 ml/min/1.73 m2), the elimination of rabeprazole is similar to that of healthy volunteers. AUC and Cmax in these patients were approximately 35% lower than in healthy volunteers. On average, T1/2 of rabeprazole was 0.82 hours in healthy volunteers, 0.95 hours in patients during hemodialysis and 3.6 hours after hemodialysis. Clearance of the drug in patients with kidney disease requiring hemodialysis was approximately twice as high as in healthy volunteers.
Chronic compensated cirrhosis
Patients with chronic compensated liver cirrhosis tolerate rabeprazole 20 mg once daily, although AUC is doubled and Cmax is increased by 50% compared to healthy volunteers.
Elderly patients
In elderly patients, the elimination of rabeprazole is somewhat slower. After 7 days of taking rabeprazole at a dose of 20 mg 1 time per day in elderly individuals, AUC was approximately twice as high and Cmax was increased by 60% compared to young healthy volunteers; There were no signs of drug accumulation.
СYР2С19 polymorphism
In patients with slow metabolism via the CYP2C19 isoenzyme, after 7 days of taking rabeprazole at a dose of 20 mg per day, the AUC increases by 1.9 times and the half-life by 1.6 times compared with the same parameters in “rapid metabolizers”, while how Cmax increases by 40%.
Khairabezol tablet p/o 20 mg N30 (Hinglas)
Khairabezol tablets should not be chewed or crushed. The tablets should be swallowed whole. It has been established that neither time of day nor food intake affects the activity of rabeprazole. For gastric ulcers in the acute stage and anastomotic ulcers, it is recommended to take 20 mg orally once a day. Usually cure occurs after 6 weeks of therapy, but in some cases the duration of treatment can be increased by 6 weeks. For duodenal ulcers in the acute stage, it is recommended to take 20 mg orally once a day. The duration of treatment is from 2 to 4 weeks. If necessary, the duration of treatment can be increased by another 4 weeks. When treating erosive gastroesophageal reflux disease (GERD) or reflux esophagitis, it is recommended to take 20 mg orally once a day. The duration of treatment is from 4 to 8 weeks. If necessary, the duration of treatment can be increased by another 8 weeks. For maintenance therapy of gastroesophageal reflux disease (GERD), it is recommended to take 10 mg or 20 mg orally once a day. The duration of treatment depends on the patient's condition. For non-erosive gastroesophageal reflux disease (NERD), it is recommended to take 10 mg or 20 mg orally once a day. If symptoms do not disappear after four weeks of treatment, the patient should be further examined. After relief of symptoms, to prevent their subsequent occurrence, the drug should be taken orally at a dose of 10 mg once a day as required. For the treatment of Zollinger-Elison syndrome and other conditions characterized by pathological hypersecretion, the dose is selected individually. The initial dose is 60 mg per day, then the dose is increased and the drug is prescribed at a dose of up to 100 mg per day with a single dose or 60 mg twice a day. For some patients, fractional dosing of the drug is preferable. Treatment should be continued as clinically necessary. In some patients with Zollinger-Ellison syndrome, the duration of treatment with rabeprazole is up to one year. For Helicobacter pylori eradication, it is recommended to take 20 mg orally twice a day according to a specific regimen with an appropriate combination of antibiotics. The duration of treatment is 7 days. Patients with renal and hepatic insufficiency No dose adjustment is required for patients with renal insufficiency. In patients with mild to moderate hepatic impairment, blood concentrations of rabeprazole are usually higher than in healthy patients. When prescribing the drug Khairabezol to patients with severe liver failure, caution should be exercised. Elderly patients No dose adjustment is required. Children The safety and effectiveness of rabeprazole in children aged 12 years and older has been established for short-term (up to 8 weeks) treatment of GERD. The recommended dose for children aged 12 years and older is 20 mg once daily for up to 8 weeks. The safety and effectiveness of rabeprazole for other indications has not been established in pediatric patients.