Esomeprazole
The drug is used orally, parenterally intravenously.
The drug in the form of film-coated tablets is used orally. The tablet should be swallowed whole with liquid. Tablets should not be chewed or broken. For patients with difficulty swallowing, you can dissolve the tablets in half a glass of still water.
Adults and children from 12 years old
Gastroesophageal reflux disease
Treatment of erosive reflux esophagitis: 40 mg once a day for 4 weeks. An additional 4-week course of treatment is recommended in cases where, after the first course, healing of esophagitis does not occur or symptoms persist.
Long-term maintenance treatment after healing of erosive reflux esophagitis to prevent relapse: 20 mg once a day.
Symptomatic treatment of gastroesophageal reflux disease: 20 mg once daily in patients without esophagitis. If symptoms do not disappear after 4 weeks of treatment, the patient should be further examined. After eliminating the symptoms, you can switch to the “as needed” regimen of the drug, i.e. take 20 mg once daily when symptoms return. For patients taking NSAIDs who are at risk of developing gastric or duodenal ulcers, treatment on an 'as needed' basis is not recommended.
Adults
Peptic ulcer of the stomach and duodenum
As part of combination therapy for Helicobacter pylori eradication:
treatment of duodenal ulcer associated with Helicobacter pylori: the drug 20 mg, amoxicillin 1 g and clarithromycin 500 mg. All drugs are taken twice a day for 1 week.
Prevention of relapse of peptic ulcers associated with Helicobacter pylori: drug 20 mg, amoxicillin 1 g and clarithromycin 500 mg. All drugs are taken twice a day for 1 week.
Long-term acid suppression therapy in patients who have suffered bleeding from a peptic ulcer (after intravenous use of drugs that reduce the secretion of gastric glands to prevent relapse) 40 mg of the drug 1 time per day for 4 weeks after the end of intravenous therapy with drugs that reduce the secretion of gastric glands.
Patients taking NSAIDs for a long time:
- healing of stomach ulcers associated with taking NSAIDs: 20 mg or 40 mg once a day. The duration of treatment is 4-8 weeks.
- prevention of gastric and duodenal ulcers associated with taking NSAIDs: 20 mg or 40 mg once a day.
Conditions associated with pathological hypersecretion of the gastric glands, including Zollinger-Ellison syndrome and idiopathic hypersecretion: The recommended starting dose is 40 mg twice daily. In the future, the dose is selected individually, the duration of treatment is determined by the clinical picture of the disease.
If it is impossible to carry out oral therapy, patients may be recommended to prescribe the drug parenterally at a dose of 20-40 mg once a day, for which a lyophilisate is used to prepare a solution for intravenous administration.
Esomeprazole, 28 pcs., 40 mg, enteric film-coated tablets
Inside.
The tablet should be swallowed whole, without chewing, with a sufficient amount of water.
GERD (adults and children over 12 years old)
Treatment of erosive reflux esophagitis:
40 mg 1 time per day for 4 weeks. An additional 4-week course of treatment is recommended in cases where, after the first course, healing of esophagitis does not occur or symptoms persist.
Long-term maintenance treatment after healing of erosive reflux esophagitis, prevention of relapses:
20 mg 1 time per day.
Symptomatic treatment of GERD:
20 mg once daily for patients without esophagitis. If symptoms do not disappear after 4 weeks of treatment, the patient should be further examined. After eliminating the symptoms, you can switch to the “as needed” regimen of the drug - 20 mg 1 time per day when symptoms return. For patients taking NSAIDs who are at risk of developing gastric or duodenal ulcers, as-needed treatment is not recommended.
Peptic ulcer of the stomach and duodenum (adults)
As part of combination therapy for Helicobacter pylori eradication:
- treatment of duodenal ulcer associated with Helicobacter pylori
: Esomeprazole Canon 20 mg, amoxicillin 1000 mg and clarithromycin 500 mg. All drugs are taken 2 times a day for 1 week;
- prevention of relapses of peptic ulcers associated with Helicobacter pylori
: Esomeprazole Canon 20 mg, amoxicillin 1000 mg and clarithromycin 500 mg. All drugs are taken 2 times a day for 1 week.
Long-term acid suppression therapy in patients who have suffered bleeding from a peptic ulcer (after intravenous use of drugs that reduce the secretion of gastric glands to prevent relapse)
: Esomeprazole Canon 40 mg 1 time per day for 4 weeks after intravenous use of drugs that reduce the secretion of gastric glands.
Patients taking NSAIDs for a long time
Treatment of gastric ulcers caused by taking NSAIDs
: Esomeprazole Canon 20 mg 1 time per day; Duration of treatment is 4–8 weeks.
Prevention of gastric and duodenal ulcers caused by taking NSAIDs
: Esomeprazole Canon 20 mg once a day.
Conditions characterized by pathological hypersecretion of the gastric glands, incl. Zollinger-Ellison syndrome and idiopathic hypersecretion
: the recommended initial dose of Esomeprazole Canon is 40 mg 2 times a day. Next, the dose is selected individually, the duration of treatment is determined by the clinical picture of the disease.
There is experience with the use of esomeprazole from 80 to 160 mg/day; when taking the drug more than 80 mg/day, it is recommended to divide the required dose into 2 doses.
Kidney failure
: No dose adjustment of Esomeprazole Canon is required. However, experience with the use of esomeprazole in patients with severe renal failure is limited, and therefore caution should be exercised when prescribing the drug to this category of patients.
Liver failure
: For mild to moderate liver failure, no dose adjustment is required. For patients with severe liver failure, the maximum daily dose should not exceed 20 mg.
Elderly patients
: No dose adjustment is required.
Esomeprazole intestinal tablet p o film 20 mg x28
MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION INSTRUCTIONS
on the medical use of the drug EZOMEPRAZOLE
Registration number: LP-005744
Trade name: Esomeprazole
International nonproprietary name: esomeprazole
Dosage form: enteric-soluble, film-coated tablets
Compound
1 film-coated tablet. 20 mg contains:
Tablet core composition:
Active ingredient: esomeprazole magnesium dihydrate - 21.7 mg (in terms of esomeprazole - 20 mg).
Excipients: , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , corn
pregelatinized, colloidal silicon dioxide, mannitol, sodium gearyl fumarate, microcrystalline cellulose, type 200, sodium carboxymethyl starch.
Tablet shell composition: hypromellose, macrogol-6000, acrylyse II yellow 4932220000 (methacrylic acid and ethyl acrylate copolymer (1:1), talc, titanium dioxide, poloxamer 407, calcium silicate, sodium bicarbonate, iron oxide yellow dye, sodium lauryl sulfate).
1 film-coated tablet. 40 mg contains:
Tablet core composition:
Active ingredient: esomeprazole magnesium dihydrate - 43.4 mg (in terms of esomeprazole - 40.0 mg).
Excipients: , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , corn
pregelatinized, colloidal silicon dioxide, mannitol, sodium gearyl fumarate, microcrystalline cellulose, type 200, sodium carboxymethyl starch.
Tablet shell composition: hypromellose, macrogol-6000, acrylyse II yellow 4932220000 (methacrylic acid and ethyl acrylate copolymer (1:1), talc, titanium dioxide, poloxamer 407, calcium silicate, sodium bicarbonate, iron oxide yellow dye, sodium lauryl sulfate).
Description
Dosage 20 mg, round, biconvex, yellow to grayish-yellow film-coated tablets, scored on one side.
Dosage 40 mg. Tablets are oval, biconvex, yellow to grayish-yellow in color, scored on one side and engraved with the f symbol on the other.
Pharmacotherapeutic group: gastric gland secretion reducing agent - proton pump inhibitor ATC code: A02BC05 Pharmacological properties Pharmacodynamics
Esomeprazole is the 5-isomer of omeprazole and reduces the secretion of hydrochloric acid in the stomach by specifically inhibiting the proton pump in the parietal cells of the gastric mucosa. The 5- and K-isomers of omeprazole have similar pharmacodynamic activities.
Mechanism of action
Esomeprazole is a weak base that is converted into an active form in the highly acidic environment of the secretory tubules of the parietal cells of the gastric mucosa, where it inhibits the proton pump - the enzyme NAA*-ATPase, thereby inhibiting both basal and stimulated secretion of hydrochloric acid.
Effect on the secretion of hydrochloric acid in the stomach.
After taking esomeprazole orally at a dose of 20 mg or 40 mg, the therapeutic effect develops within 1 hour. When taking the drug daily for 5 days at a dose of 20 mg once a day, the average maximum acid production after stimulation with pentagastrin is reduced by 90% (when measured 6-7 hours after taking the drug on the 5th day of therapy).
In patients with gastroesophageal reflux disease (GERD) and the presence of clinical symptoms, after 5 days of daily oral esomeprazole 20 mg or 40 mg, intragastric pH values above 4 were maintained for an average of 13 and 17 hours out of 24 hours. While taking esomeprazole at a dose of 20 mg per day, intragastric pH above 4 was maintained for at least 8.12 and 16 hours in 76%, 54% and 24% of patients, respectively. For 40 mg esomeprazole, this ratio is 97%, 92% and 56%, respectively.
A correlation was found between the concentration of the drug in plasma and the inhibition of hydrochloric acid secretion (the A11C parameter, the area under the concentration-time curve, was used to assess the concentration).
The therapeutic effect achieved by inhibiting the secretion of hydrochloric acid.
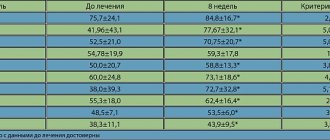
When taking esomeprazole at a dose of 40 mg, healing of reflux esophagitis occurs in approximately 78% of patients after 4 weeks of therapy and in 93% after 8 weeks of therapy.
Treatment with esomeprazole at a dose of 20 mg twice daily in combination with appropriate antibiotics for one week leads to successful eradication of Hexobacteria in approximately 90% of patients.
Patients with uncomplicated duodenal ulcer after a weekly course of eradication therapy do not require subsequent monotherapy with drugs that reduce the secretion of gastric glands to heal the ulcer and eliminate symptoms.
The effectiveness of esomeprazole for peptic ulcer bleeding was demonstrated in a study of patients with endoscopically confirmed peptic ulcer bleeding.
Other effects associated with inhibition of hydrochloric acid secretion
During treatment with drugs that reduce the secretion of gastric glands, the concentration of gastrin in the plasma increases as a result of a decrease in acid secretion. Due to a decrease in the secretion of hydrochloric acid, the concentration of chromogranin A (CgA) increases. Increased concentrations of SdA may affect the results of examinations for the detection of neuroendocrine tumors. To prevent this effect, therapy with proton pump inhibitors should be suspended 5-14 days before testing the concentration of SdA. If during this time the SdA concentration has not returned to normal, the study should be repeated.
In children and adult patients receiving esomeprazole for a long time, there is an increase in the number of enterochromaffin-like cells, probably associated with an increase in plasma gastrin concentrations. This phenomenon has no clinical significance.
Patients taking drugs that reduce the secretion of gastric glands for a long period of time are more likely to develop glandular cysts in the stomach. These phenomena are caused by physiological changes as a result of pronounced inhibition of hydrochloric acid secretion. Cysts are benign and undergo reverse development.
The use of drugs that suppress the secretion of hydrochloric acid in the stomach, including proton pump inhibitors, is accompanied by an increase in the content of microbial flora in the stomach that is normally present in the gastrointestinal tract. The use of proton pump inhibitors may lead to a slight increase in the risk of infectious diseases of the gastrointestinal tract caused by bacteria of the genus S. and CatrutobacTer 5pp., and in hospitalized patients, it is likely that C1oIpsitum suricie is present.
In two comparative studies with ranitidine as the active comparator, esomeprazole was shown to be superior in healing gastric ulcers in patients treated with nonsteroidal anti-inflammatory drugs (NSAIDs), including selective cyclooxygenase-2 (COX-2) inhibitors.
In two studies, esomeprazole was highly effective in preventing gastric and duodenal ulcers in patients treated with NSAIDs (age group over 60 years and/or with a history of peptic ulcers), including selective COX-2 inhibitors.
Pharmacokinetics Absorption and distribution
Esomeprazole is unstable in an acidic environment, therefore, for oral administration, tablets containing granules of the drug are used that are resistant to the action of gastric juice. Under conditions, only a small part of esomeprazole is converted into the K-isomer. The drug is rapidly absorbed: maximum plasma concentration is achieved 1-2 hours after administration. The absolute bioavailability of esomeprazole after a single dose of 40 mg is 64% and increases to 89% with daily dosing once daily. For a dose of 20 mg esomeprazole, these figures are 50% and 68%, respectively. The volume of distribution at steady state concentration in healthy people is approximately 0.22 l/kg body weight. Esomeprazole is 97% bound to plasma proteins.
Eating slows down and reduces the absorption of esomeprazole in the stomach, but this does not have a significant effect on the effectiveness of inhibition of hydrochloric acid secretion.
Metabolism and excretion
Esomeprazole is metabolized via the cytochrome P450 system. The main part is metabolized with the participation of a specific polymorphic isoenzyme CYP2C19, resulting in the formation of hydroxylated and demethylated metabolites of esomeprazole. Metabolism of the remaining part is carried out by the CURZA4 isoenzyme, resulting in the formation of a sulfo derivative of esomeprazole, which is the main metabolite detected in plasma.
The parameters given below mainly reflect the nature of pharmacokinetics in patients with increased activity of the CYP2C19 isoenzyme.
The total clearance is approximately 17 l/h after a single dose of the drug and 9 l/h after repeated doses. The half-life is 1.3 hours when taken systematically once a day. ACV increases with repeated administration of esomeprazole. The dose-dependent increase in ACV upon repeated administration of esomeprazole is nonlinear, which is a consequence of a decrease in first-pass metabolism through the liver, as well as a decrease in systemic clearance, probably caused by inhibition of the CYP2C19 isoenzyme by esomeprazole and/or its sulfo derivatives. When taken daily once a day, esomeprazole is completely eliminated from the blood plasma in the interval between doses and does not accumulate. The main metabolites of esomeprazole do not affect the secretion of gastric acid. When administered orally, up to 80% of the dose is excreted in the form of metabolites in the urine, the rest is excreted through the intestines. Less than 1% of unchanged esomeprazole is found in urine.
Features of pharmacokinetics in some groups of patients
Approximately 2.9±1.5% of the population has reduced activity of the CYP2C19 isoenzyme. In such patients, the metabolism of esomeprazole is mainly carried out as a result of the action of CYP3A4. When systematically taking 40 mg of esomeprazole once a day, the average ACS value is 100% higher than the value of this parameter in patients with increased activity of the CYP2C19 isoenzyme. The average values of maximum plasma concentrations in patients with reduced isoenzyme activity are increased by approximately 60%. These features do not affect the dose and method of administration of esomeprazole.
In elderly patients (71-80 years), the metabolism of esomeprazole does not undergo significant changes.
After a single dose of 40 mg of esomeprazole, the average ACV value in women is 30% higher than that in men. When taking the drug daily once a day, there are no differences in pharmacokinetics between men and women. These features do not affect the dose and method of administration of esomeprazole.
In patients with mild to moderate hepatic impairment, the metabolism of esomeprazole may be impaired. In patients with severe liver failure, the metabolic rate is reduced, which leads to an increase in the ACS value for esomeprazole by 2 times.