Nosological classification (ICD-10)
- C25 Malignant neoplasm of the pancreas
- E84.1 Cystic fibrosis with intestinal manifestations
- K31.5 Duodenal obstruction
- K31.8 Other specified diseases of the stomach and duodenum
- K74 Fibrosis and cirrhosis of the liver
- K83.1 Bile duct obstruction
- K86.1 Other chronic pancreatitis
- K86.8 Other specified diseases of the pancreas
- K86.8.0* Hypofunction of the pancreas, exocrine
- K91 Digestive disorders following medical procedures, not elsewhere classified
- K91.5 Postcholecystectomy syndrome
- K92.8 Other specified diseases of the digestive system
- R54 Old age
- Z100* CLASS XXII Surgical practice
Composition and release form
Creon® 10000
| Capsules | 1 caps. |
| pancreatin | 150 mg |
| amylase | 8000 EF units |
| lipase | 10000 EF units |
| proteases | 600 units EF |
| excipients: macrogol; liquid paraffin; methylhydroxypropylcellulose phthalate; dimethicone 1000; dibutyl phthalate | |
| capsule shell: red iron oxide (E172); iron oxide black (E172); iron oxide yellow (E172); titanium dioxide (E171); gelatin |
10 pcs in blister; in a cardboard pack there are 2 blisters; 25 pcs in blister; in a cardboard pack there are 2 or 4 blisters; in polyethylene bottles of 20 and 50 pcs.; 1 bottle in a cardboard pack.
Creon® 25000
| Capsules | 1 caps. |
| pancreatin | 300 mg |
| amylase | 18000 EF units |
| lipase | 25000 EF units |
| proteases | 1000 units EF |
| excipients: macrogol 4000; liquid paraffin, methylhydroxypropylcellulose phthalate; dimethicone; dibutyl phthalate | |
| capsule shell: red iron oxide (E172); iron oxide yellow (E172); titanium dioxide (E171); gelatin |
10 pcs in blister; in a cardboard pack there are 2 blisters; 25 pcs in blister; in a cardboard pack there are 2 or 4 blisters; in polyethylene bottles of 20 and 50 pcs.; 1 bottle in a cardboard pack.
Pharmacodynamics
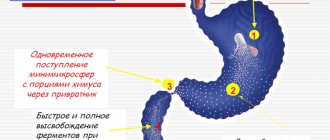
An enzyme preparation that improves digestion processes. Pancreatic enzymes included in the drug facilitate the breakdown of proteins, fats, and carbohydrates, which leads to their complete absorption in the small intestine. Capsules containing enteric-coated mini-microspheres dissolve quickly in the stomach, releasing hundreds of mini-microspheres. The purpose of the multi-unit dose principle is to mix the mini-microspheres with the intestinal contents, and ultimately to better distribute the enzymes after their release within the intestinal contents.
Creon 10000 capsules No. 20
Creon 10000 capsules No. 20
Instructions for use Creon 10000 caps. No. 20
Dosage forms capsules 10000 units Synonyms Gastenorm forte Gastenorm forte 1000
Mezim 20000
Mikrasim Pangrol 10000 Pangrol 25000 Panzinorm 10000 Panzinorm forte 20000 Panzinorm forte-N Pancreazim Pancreatin Pancreatin forte Pancreatin-LekT Penzital Enzistal-P Ermital Group Enzyme agents that improve digestive processes International nonproprietary name Pancreatin Composition Active ingredient - pancreatin 150 mg, which corresponds to the content: lipase 10,000 units, amylase 8,000 units, protease 600. Manufacturers Abbott Products GmbH (Germany) Pharmacological action An enzyme preparation that improves digestive processes. Pancreatic enzymes included in the drug facilitate the digestion of proteins, fats, and carbohydrates, which leads to their complete absorption in the small intestine. The drug contains pancreatin in the form of mini-microspheres, enteric-coated, which quickly dissolve in the stomach, releasing hundreds of mini-microspheres. The purpose of this principle is to mix the mini-microspheres with the intestinal contents and, ultimately, to better distribute the enzymes after their release within the intestinal contents. When minimicrospheres reach the small intestine, the enteric coating is destroyed (at pH>5.5), pancreatic enzymes with lipolytic, amylolytic and proteolytic activity are released, which leads to the breakdown of fats, carbohydrates and proteins. The resulting substances are then either directly absorbed or further hydrolyzed by intestinal enzymes. Animal studies have demonstrated a lack of absorption of intact (uncleaved) enzymes and, as a consequence, classical pharmacokinetic studies have not been performed. Drugs containing pancreatic enzymes do not require absorption to exert their effects. On the contrary, the full therapeutic activity of these drugs is realized in the lumen of the gastrointestinal tract. Moreover, in their chemical structure they are proteins and, in connection with this, when passing through the gastrointestinal tract, they undergo proteolytic cleavage until they are absorbed in the form of peptides and amino acids. effects Gastrointestinal disorders. Very common (>=1/10): pain in the abdominal area. Often (>= 1/100, =1/1000, Indications for use Replacement therapy for insufficiency of exocrine pancreatic function in children and adults, caused by various diseases of the gastrointestinal tract and most often found in: cystic fibrosis; chronic pancreatitis; after pancreatic surgery gland; after gastrectomy; pancreatic cancer; partial gastrectomy (for example, Billroth II); obstruction of the pancreatic ducts or common bile duct (for example, due to a neoplasm); Shwachman-Diamond syndrome; condition after an attack of acute pancreatitis and resumption of nutrition. Contraindications Increased sensitivity to any of the components of the drug. Method of administration and dosage Orally. Doses of the drug are selected individually depending on the severity of the disease and the composition of the diet. Capsules should be taken during or immediately after each meal (including light snacks), swallowed whole, do not break or chew with sufficient liquid. If swallowing is difficult (for example, in small children or elderly patients), the capsules are carefully opened, and minimicrospheres are added to soft food that does not require chewing and has a sour taste (pH Overdose Symptoms: hyperuricuria and hyperuricemia. Treatment: discontinuation of the drug, symptomatic therapy. Interaction No studies have been conducted. Special instructions In patients with cystic fibrosis who received high doses of pancreatin preparations, strictures of the ileum, cecum and colon (fibrosing colonopathy) have been described. As a precaution, if unusual symptoms or changes in the abdominal cavity occur, medical examination is necessary to rule out fibrosing colonopathy, especially in patients taking the drug at a dose of more than 10,000 lipase units/kg per day. Like all currently used porcine pancreatin preparations, the drug is made from pancreatic tissue from pigs specially raised for human consumption. Although the potential for transmission of an infectious agent to humans has been minimized by testing and inactivation of certain viruses during the manufacturing process, there is a theoretical risk of transmission of viral disease, including diseases caused by new or unknown viruses. The presence of swine viruses that can infect humans cannot be completely excluded. However, over a long period of time using porcine pancreas extracts, not a single case of infectious disease transmission has been recorded. Storage conditions The drug should be stored out of the reach of children at a temperature not exceeding 25 C in tightly closed packaging.
Indications of the drug Creon® 10000
replacement therapy for insufficiency of exocrine pancreatic function in cystic fibrosis, chronic pancreatitis, pancreatectomy, pancreatic cancer, duct obstruction (pancreatic duct or common bile duct) due to neoplasm, Shwachman-Diamond syndrome, in old age;
symptomatic treatment of digestive disorders during partial gastrectomy (Billroth-I/II), total gastrectomy; after cholecystectomy, with duodeno- and gastrostasis, biliary obstruction, cholestatic hepatitis, liver cirrhosis, pathology of the terminal part of the small intestine, excessive bacterial growth in the small intestine.
Creon 10000 20 pcs. enteric capsules abbott laboratories gmbh/
pharmachologic effect
Digestive enzyme agent.
Composition and release form Creon 10000 20 pcs. enteric capsules abbott laboratories gmbh/
Capsules - 1 capsule:
- Active substance: pancreatin - 150 mg (which corresponds to 10,000 IU of Eur.F. lipase, 8,000 IU of Eur.F. amylase, 600 IU of Eur.F. protease.);
- Excipients: macrogol 4000 - 37.50 mg, hypromellose phthalate - 56.34 mg, dimethicone 1000 - 1.35 mg, cetyl alcohol - 1.18 mg, triethyl citrate - 3.13 mg;
- Hard gelatin capsule: gelatin - 60.44 mg, red iron oxide dye (E 172) - 0.23 mg, yellow iron oxide dye (E 172) - 0.05 mg, black iron oxide dye (E 172) - 0, 09 mg, titanium dioxide (E 171) - 0.07 mg, sodium lauryl sulfate - 0.12 mg.
20, 50 or 100 capsules in a white high-density polyethylene bottle with a polypropylene screw cap and tamper evident. A label is placed on the bottle. 1 bottle along with instructions for use in a cardboard box.
Description of the dosage form
Hard gelatin capsules No. 2, consisting of a brown opaque cap and a transparent colorless body.
The contents of the capsules are minimicrospheres of light brown color.
Directions for use and doses
Creon is taken orally. The dose of the drug depends on the age and degree of pancreatic insufficiency and is calculated in terms of lipase enzyme.
The average dose for adults is 150,000 units of lipase/day. In case of complete insufficiency of pancreatic function - 400,000 units/day, which corresponds to the daily requirement of an adult for lipase. The maximum daily dose is 15,000-20,000 units/kg of weight.
Children under 1.5 years of age are prescribed 50,000 units/day; over 1.5 years - 100,000 units/day. Capsules are taken with meals, swallowed whole with plenty of non-alkaline liquid (water, fruit juices).
Pharmacodynamics
An enzyme preparation that improves digestion processes. Pancreatic enzymes included in the drug facilitate the breakdown of proteins, fats, and carbohydrates, which leads to their complete absorption in the small intestine. Capsules containing enteric-coated mini-microspheres dissolve quickly in the stomach, releasing hundreds of mini-microspheres. The purpose of the multi-unit dose principle is to mix the mini-microspheres with the intestinal contents, and ultimately to better distribute the enzymes after their release within the intestinal contents.
Pharmacokinetics
When the mini-microspheres reach the small intestine, the enteric coating is destroyed, releasing enzymes with lipolytic, amylolytic and proteolytic activity, which ensure the breakdown of fats, starches and proteins.
Indications for use Creon 10000 20 pcs. enteric capsules abbott laboratories gmbh/
Creon is an enzyme preparation that improves digestion processes. Creon is used as replacement therapy for insufficiency of exocrine pancreatic function, for symptomatic treatment of digestive disorders in the following cases: conditions after cholecystectomy, partial resection of the stomach, total gastrectomy, duodeno- and gastrostasis, biliary obstruction, cholestatic hepatitis, cirrhosis of the liver, pathology of the terminal part of the small intestines, bacterial overgrowth in the small intestine.
Contraindications
Creon should not be taken in the early stages of acute pancreatitis, as well as in case of hypersensitivity to porcine pancreatin or to any other component of the drug.
Application Creon 10000 20 pcs. enteric capsules abbott laboratories gmbh/ during pregnancy and breastfeeding
The use of Creon is possible under the supervision of a physician.
special instructions
In patients with cystic fibrosis who received high doses of pancreatin preparations, strictures of the ileum, cecum and colon (fibrosing colonopathy) have been described. As a precaution, if unusual symptoms or changes in the abdominal cavity occur, medical examination is necessary to rule out fibrosing colonopathy, especially in patients taking the drug at a dose of more than 10,000 lipase units/kg per day.
Impact on the ability to drive vehicles and operate machinery
The use of the drug Krson® 10000 does not affect or has an insignificant effect on the ability to drive a car and operate machinery.
Overdose
Symptoms: hyperuricuria, hyperuricemia. Treatment: drug withdrawal, symptomatic therapy.
Side effects Creon 10000 20 pcs. enteric capsules abbott laboratories gmbh/
When using Creon, adverse reactions may occur, such as diarrhea, nausea, and allergic reactions.
Drug interactions
There are no reports of interaction with other drugs.
Directions for use and doses
Inside. The dose is selected individually depending on the severity of the disease and the composition of the diet. It is recommended to take 1/3 or 1/2 of a single dose at the beginning of a meal, and the rest during meals. If swallowing is difficult (for example, in small children or elderly patients), the capsules are carefully opened, and mini-microspheres are added to liquid food that does not require chewing, or taken with liquid. Any mixture of mini-microspheres with food or liquid cannot be stored and should be taken immediately after preparation. Crushing or chewing mini-microspheres, as well as adding them to food with a pH above 5.5, leads to the destruction of their shell, which protects against the action of gastric juice.
For cystic fibrosis, the dose depends on body weight and is at the beginning of treatment in children under 4 years of age - 1000 lipase units / kg for each meal, over 4 years - 500 lipase units / kg during meals. The dose depends on the severity of symptoms of the disease, control of steatorrhea and maintenance of good nutritional status. In most patients, the dose should not exceed 10,000 units/kg/day.
For other conditions accompanied by exocrine pancreatic insufficiency, the dose is set taking into account the individual characteristics of the patient (degree of digestive insufficiency, fat content in food). The dose with the main meal (breakfast, lunch and dinner) is 20,000–75,000 IU of EP lipase, while taking a light snack - 5,000–25,000 IU of EP lipase.
The usual initial dosage of Creon® is 10,000–25,000 IU of EP lipase with the main meal. To reduce steatorrhea and maintain optimal patient condition, the dose may be increased. According to usual clinical practice, the patient should receive at least 20,000–50,000 units of EP lipase with food.
Creon 10000 capsules 10000 units bottle 20 pcs.
Inside. Doses of the drug are selected individually depending on the severity of the disease and the composition of the diet. Capsules should be taken during or immediately after each meal (including light snacks), swallowed whole, do not break or chew, with a sufficient amount of liquid. For difficult When swallowed (for example, in young children or elderly patients), the capsules are carefully opened and the minimicrospheres are added to soft, non-chewable food that has a sour taste (pH < 5.5), or taken with a liquid that also has a sour taste (pH < 5.5). 5.5). For example, minimicrospheres can be added to applesauce, yogurt, or fruit juice (apple, orange, or pineapple) with a pH less than 5.5. It is not recommended to add the contents of the capsules to hot food. Any mixture of minimicrospheres with food or liquid cannot be stored and should be taken immediately after preparation. Crushing or chewing minimicrospheres, or mixing them with food or liquid with a pH greater than 5.5, can destroy their protective enteric coating. This can lead to early release of enzymes in the mouth, reduced effectiveness and irritation of the mucous membranes. It is necessary to ensure that there are no mini-microspheres left in the mouth. It is important to ensure that the patient has sufficient and constant fluid intake, especially if there is increased fluid loss. Inadequate fluid intake may lead to or worsen constipation. Dose for adults and children with cystic fibrosis. The dose depends on body weight and should be 1000 lipase units/kg at each meal for children under four years of age at the beginning of treatment, and 500 lipase units/ kg at mealtime for children over four years of age and adults. The dose should be determined depending on the severity of symptoms of the disease, control of steatorrhea and maintenance of adequate nutritional status. In most patients, the dose should remain less than or not exceed 10,000 lipase units/kg body weight per day or 4000 lipase units/g of fat consumed. Dose for other conditions accompanied by exocrine pancreatic insufficiency. The dose should be set taking into account the individual characteristics of the patient, which include the degree of digestive insufficiency and the fat content of food. The dose that the patient requires along with the main meal varies from 25,000 to 80,000 IU of lipase, and while taking a light snack - half the individual dose. In children, the drug should be used in accordance with the doctor's prescription.
special instructions
Strictures of the ileum and cecum and colitis have been described in patients with cystic fibrosis who received high doses of pancreatin preparations. In case-control studies, there was no evidence of a relationship with Creon and the occurrence of fibrosing colonopathy. As a precaution to exclude colonic involvement in patients with cystic fibrosis, it is recommended to monitor any unusual symptoms or changes in the abdominal cavity - especially if the patient is taking more than 10,000 IU lipase/kg/day.
The drug does not affect the ability to drive a car or control machines and mechanisms.
Creon 10000, enteric capsules 10000 units, 20 pcs.
The drug is taken orally. The dose is selected individually, depending on the severity of the disease and the composition of the diet.
Capsules should be taken during or immediately after each meal (including snacks), swallowed whole, not broken or chewed, and washed down with sufficient liquid.
If swallowing is difficult (for example, in small children or elderly patients), the capsules are carefully opened, and the minimicrospheres are added to soft food that does not require chewing and has a sour taste (pH < 5.5), or taken with a liquid that also has a sour taste (pH < 5.5). For example, minimicrospheres can be added to applesauce, yogurt, or fruit juice (apple, orange, or pineapple) with a pH less than 5.5. It is not recommended to add the contents of the capsules to hot food. Any mixture of minimicrospheres with food or liquid cannot be stored and should be taken immediately after preparation.
Crushing or chewing minimicrospheres, as well as mixing them with food or liquid with a pH greater than 5.5, can destroy their protective enteric coating. This can lead to early release of enzymes in the mouth, reduced effectiveness and irritation of the mucous membranes. You need to make sure that there are no mini-microspheres left in your mouth.
It is important to ensure that the patient maintains adequate fluid intake, especially if there is increased fluid loss. Inadequate fluid intake may cause or worsen constipation.
Cystic fibrosis
The dose for adults and children depends on body weight and should be at the beginning of treatment 1000 lipase units/kg per meal for children under 4 years of age , and 500 lipase units/kg per meal for children over 4 years of age and adults .
The dose should be determined depending on the severity of symptoms of the disease, control of steatorrhea and maintenance of adequate nutritional status.
For most patients, the dose should remain less than or not exceed 10,000 lipase units/kg body weight per day or 4,000 lipase units/g fat intake.
Other conditions associated with exocrine pancreatic insufficiency
The dose should be set taking into account the individual characteristics of the patient, which include the degree of digestive insufficiency and the fat content of food. The dose that the patient requires along with the main meal varies from 25,000 to 80,000 units of lipase, and while taking a snack - half the individual dose.
The dose to improve food digestion in patients with normal gastrointestinal function in cases of dietary errors depends on body weight and fat content in food, varies from 10,000 to 20,000 IU of lipase per dose.
In children, the drug should be used as prescribed by a doctor.