Neobutin and Neobutin Retard
A structural analogue of Trimedat - Neobutin, which is produced in India and the Russian Federation, is tableted, has 100 mg of the active substance trimebutine maleate in the base. Indications for use and contraindications are identical.
The drug is recommended for irritable bowel syndrome, incomplete obstruction of the bile ducts. It is prohibited for use by children under three years of age, allergies to components, while pregnant, breastfeeding, or with intolerance to galactose and glucose.
Neobutin is prescribed before meals, three times a day, the dose is correlated with the patient’s age:
- from three to five years – 25 mg;
- up to 12 years – 50 mg;
- over 12 – 100-200 mg depending on weight, individual characteristics, and form of the disease.
Negative effects during use are limited to dyspepsia, dry mucous membranes, drowsiness, cephalalgia, vertigo, allergies, menstrual irregularities, engorgement of the mammary glands, and urinary retention.
Trimedat Forte as a substitute involves the use of Neobutin Retard - tablets with 300 mg of active principle, similar indications, contraindications and dosing. The medicine can be used in adults and children on the recommendation of a doctor.
The price of Neobutin is 209 rubles, Neobutin-Retard is 396.
Trimebutine
Trimebutine
(lat.
Trimebutine
) - a drug, myotropic antispasmodic, regulator of motility of the gastrointestinal tract. Some gastroenterologists also classify trimebutine as prokinetics (Alekseeva E.V. et al.). It is often used as a salt of maleic acid (trimebutine maleate). Trimebutine maleate is an encephalic ligand of the receptor [2-(dimethylamino)-2-phenylbutyl ester of 3,4,5-trimethoxybenzoic acid] acts as an agonist of peripheral μ, k- and δ-opiate receptors, triggering phase III of the motor complex (Nizhevich A .A., etc.).
Trimebutine is a chemical compound
Chemically, trimebutine is 2-(dimethylamino)-2-phenylbutyl ester of 3,4,5-trimethoxybenzoic acid. The empirical formula of trimebutine is C22H29NO5.
Trimebutine is a drug
Trimebutine is the international nonproprietary name (INN) of the drug.
According to the pharmacological index, trimebutine belongs to the groups “Myotropic antispasmodics” and “Gastrointestinal motility stimulants, including emetics”. According to ATC - to the group “Drugs for the treatment of functional intestinal disorders”, subgroup “Synthetic anticholinergic blockers - esters with a tertiary amino group” and has code A03AA05. Regulation of gastrointestinal motility is ensured by the interaction of nervous and humoral mechanisms. At the level of the intestinal wall, intramural and intercalary neurons are united into plexuses - Meissnerian and Auerbachian, consisting of approximately 108 nerve cells or 20,000 neurons per 1 cm2 of intestinal surface. The neurotransmitters of the enteric nervous system are acetylcholine (pre- and postganglionic parasympathetic neurons), norepinephrine (postganglionic sympathetic neurons), serotonin (serotonergic neurons), dopamine (dopaminergic neurons), nitric oxide (non-adrenergic noncholinergic neurons), ATP (purinergic neurons) and enkephalins ( encephalic neurons). The latter are ligands of peripheral opiate receptors of 3 main types (c, b, k), located on cells throughout the gastrointestinal tract, including smooth muscles. Trimebutine is an agonist of peripheral opiate receptors of these types, acting throughout the gastrointestinal tract. The result of this agonism to all 3 types of receptors is the dual (regulating) effect of trimebutine on the motility of the digestive tract. It also affects the visceral sensitivity of the gastrointestinal tract, providing a moderate analgesic effect, in particular in IBS. This effect is based on the influence of the drug on the antinociceptive system of the body with an increase in the threshold of pain sensitivity, modification of pain assessment, and a decrease in the sensitivity of receptors to inflammatory mediators. Moreover, trimebutine has a local anesthetic effect that is 17 times greater than lidocaine. Trimebutine also affects the humoral regulation of gastrointestinal motility, increasing the secretion of motilin and reducing the level of gastrin, enteroglucagon, pancreatic polypeptide, insulin and vasoactive intensinal polypeptide (Belmer S.V. et al.).
Trimebutine maleate is used in many Western countries to treat functional gastrointestinal disorders. The mechanism of action of trimebutine maleate in patients with functional dyspepsia is not well understood. It has been suggested that the association of the weak opioid properties of trimebutine maleate is associated with sodium channel blockade and the pronounced local analgesic properties explain the effectiveness of trimebutine maleate in the treatment of abdominal pain. At the same time, through opiate receptors, trimebutine maleate mediates the release of gastrointestinal peptides, such as motilin, and modulates the release of other peptides, including vasoactive intestinal peptide, gastrin and glucagon. Trimebutine maleate has a high toxicological safety profile and demonstrates excellent tolerability of the drug. There is no evidence that trimebutine maleate has effects on the central nervous system or crosses the blood-brain barrier. The main indication for the use of trimebutine maleate is dyspeptic disorders in gastroduodenal diseases (abdominal pain, indigestion, nausea, vomiting), as well as irritable bowel syndrome, gastroesophageal reflux disease (Nizhevich A.A. et al.).
Use of trimebutine by pregnant and nursing mothers
Trimebutin is not recommended during the first trimester of pregnancy and for breastfeeding mothers.
Publications for specialists concerning the use of trimebutine in medicine and veterinary medicine
- Akopyan A.N., Belmer S.V., Vykhristyuk O.F. and others. Gastroesophageal reflux and motility disorders of the gastrointestinal tract // Doctor.ru. Pediatrics. Gastroenterology. 2014. No. 11 (99). pp. 45-49.
- Tropskaya N.S., Popova T.S. Trimebutine in the correction of disturbances in the electrical activity of the gastrointestinal tract during experimental endotoxemia // RZHGGK. – 2009. – T.19. – No. 2. – pp. 37–42.
- Belmer S.V., Gasilina T.V., Kovalenko A.A., Karpina L.M. Modern ways of correcting functional disorders of the digestive organs in children // Questions of children's dietology. – 2011. – T. 9. – No. 2. – p. 10–14.
- Uspensky Yu.P., Baryshnikova N.V., Belousova L.N. The effectiveness of trimebutine in relieving abdominal pain syndrome: analysis of PEGEG data // XX Ros. Gastroenterology Week October 6-8. RZHGGK. 2014. No. 5. P. 134.
- Narinskaya N.M. The role of functional disorders of intestinal motility in children with atopic dermatitis. Abstract of dissertation. PhD, 01/14/10 - Skin and venereal diseases. RNIMU named after. N.I. Pirogova, Moscow, 2021.
- Korotky N.G., Narinskaya N.M., Belmer S.V., Ardatskaya M.D. Correction of functional disorders of the motility of the digestive organs in atopic dermatitis in children // Questions of pediatric dietology. 2015. T. 13. No. 4. pp. 5-10.
- Pidzhimyan V.P. The influence of smoking status and the degree of nicotine dependence on the clinical and functional features of the course of GERD, the possibility of using trimebutine in complex therapy. Abstract of dissertation. Ph.D., 01/14/04 – ext. diseases. St. Petersburg State Medical University, St. Petersburg, 2017.
- Zohirov A.N. The effect of transcranial electrical stimulation on the contractile function of the gastrointestinal tract of animals. Abstract of dissertation. Ph.D., 03.03.01 – physiology. KGSHA named after. I.I. Ivanova, Kursk, 2014.
- Sein O.B., Zohirov A.N. The influence of the modeling effects of trimebutine on intestinal motility in dogs // Bulletin of the Kursk State Agricultural Academy. 2014. No. 5. pp. 67–69.
- Shaporova N.L., Pidzhimyan V.P., Dudina O.V., Yablonskaya V.N., Sarkisyan S.R. The use of trimebutine in the complex therapy of GERD in smoking patients // Bulletin of the Russian Military Medical Academy. Clinical researches. – 2021. – 1 (53). pp. 67-71.
- Nizhevich A.A., Valeeva D.S., Sataev V.U., Gafurova K.A., Akhmadeeva E.N., Akhmetshin R.Z. Modern approaches to the treatment of functional dyspepsia in childhood // Questions of pediatric dietology. 2021. T. 15. No. 3. pp. 5-11.
- Plotnikova E.Yu., Gracheva T.Yu. The place of trimebutine in the conservative treatment of sphincter of Oddi dysfunction // Attending physician. - 2018. - No. 2.
- Andreev D.N., Dicheva D.T. Optimizing treatment for patients with irritable bowel syndrome: focus on improving compliance. Medical advice. 2019; 3: 118-124.
- Belmer S.V., Abuzin M.N., Donyush E.K., Malkova O.V., Kondrashova Z.A., Boyarchuk N.G., M.D. Ardatskaya. Gastrointestinal motility in children with chronic immune thrombocytopenic purpura. Pediatrics. 2019;98(6):23–30.
- Pakhomova I.G. Motility disorders in functional gastrointestinal disorders. Possibilities of therapeutic correction based on a clinical example. Medical advice. 2020;(5):18–23.
On the website GastroScan.ru in the “Literature” section there is a subsection “Anspasmolytics”, containing publications for healthcare professionals that address the use of antispasmodics in the treatment of diseases of the gastrointestinal tract.
Videos (reports and lectures) for healthcare professionals regarding the use of trimebutine
| Belmer S.V. Prospects for electrogastroenterography in pediatrics: achievements and problems | |
| Minushkin O.N. Functional dyspepsia - a look at the problem, diagnosis, treatment | |
| Minushkin O.N. Prokinetics in gastroenterological practice |
Video for medical university students
Frame “Trimebutin” from a video lecture for 3rd year students of the Faculty of Medicine of PSPbSMU named after.
acad. I.P. Pavlova: Melnikov K.N. Drugs affecting the gastrointestinal tract On the website in the “Video” section there is a subsection for patients “Popular Gastroenterology” and subsections “For doctors” and “For medical students and residents”, containing video recordings of reports, lectures, webinars in various areas of gastroenterology for healthcare professionals and medical students.
Trade names of drugs with the active ingredient trimebutine
Medicines with the active ingredient trimebutine, registered (or having been registered in the past) in Russia: Neobutin (manufactured by JSC Pharmaceutical Enterprise Obolenskoye), Neobutin Retard (Samil Pharm Co., Ltd, Korea and JSC Pharmaceutical Enterprise Obolenskoye) ), Trimebutin SZ (Severnaya Zvezda JSC), Trimedat (Valenta Pharm JSC), Trimedat Valenta (Valenta Pharm PJSC), Trimedat Forte (Valenta Pharm PJSC).
The drug Trimpa 200 produced by McLeods, India and others is presented on the Ukrainian and Kazakh pharmaceutical markets.
Trimebutine is not approved for use in the United States. In some countries of the European Union, trimebutine is sold under the trade names Debricalm, Debridat, Digerent, Garapepsin, Ircolon, Recutin, Polybutin, Transacalm, Tribux and others. In Canada: Apo-Trimebutine and Modulon. In Japan: Butikinon, Cerekinon, Mebucolon, Mebutit, Nepten, Sakion, Supeslon, Tefmetin, VeM and others.
Official instructions for the use of drugs with the active ingredient trimebutine
- “Instructions for the medical use of the drug Trimedat” (pdf), approved by Roszdravnadzor on December 28, 2007.
- “Instructions for use of the drug for medical use Trimedat Valenta” (pdf), approved by the Ministry of Health of Russia on July 8, 2014.
- “Instructions for use of the drug for medical use Neobutin” (pdf), approved by the Ministry of Health of Russia on July 20, 2015.
- “Instructions for use of the drug for medical use NEOBUTIN retard” (pdf), approved by the Ministry of Health of Russia on September 22, 2015.
- “Instructions for use of the drug Trimedat forte” (pdf), approved by the Ministry of Health of Russia on December 20, 2017.
- “Instructions for use of the drug Trimebutin-SZ” (pdf), approved by the Ministry of Health of Russia on December 3, 2018.
Trimebutine has contraindications, side effects and application features; consultation with a specialist is necessary. Back to section
Duspatalin
A popular analogue of Trimedat is Duspatalin. This product is based on a different active base – mebeverine hydrochloride. Available in tablets of 135 mg of active ingredient and capsules of 200 mg. The essence of the action is to minimize intestinal spasms: Duspatalin inhibits the penetration of calcium into the muscles of the digestive system, blocks the connection with proteins, which automatically relaxes smooth muscles.
At the same time, the drug relieves spasm from the sphincter of any organ of the biliary system of the liver, which stimulates the digestion of food entering the stomach.
Indications for use are: irritable bowel syndrome, abdominal pain, flatulence, cholecystitis, cholestasis, colic, dyspepsia, correction of stool consistency. Duspatalin is prescribed from the age of 18, in exceptional cases - from the age of 10. Contraindicated in case of individual intolerance to components, lactose, galactose deficiency, during pregnancy and lactation.
Drink Duspatalin tablets and capsules, 1 piece, twice a day, without chewing, since the shell guarantees prolonged action.
Side effects: allergies, anaphylaxis, dyspepsia, nausea, epigastric pain, cephalalgia, insomnia, chronic fatigue syndrome, vertigo.
Price – 545 rubles.
Introduction
Irritable bowel syndrome (IBS) is a functional disorder of the gastrointestinal tract (GIT), manifested by periodic abdominal pain associated with a change in the frequency of bowel movements and/or a change in stool consistency [1, 2].
The variable pattern of the clinical course of IBS determines the use of a wide range of pharmacological drugs to correct this disorder, including antispasmodics, laxatives, antidiarrheal drugs, antifoams, pre- and probiotics [3–6]. Moreover, in clinical practice, the choice of the optimal treatment regimen that provides a long period of relapse-free remission of IBS often turns out to be a difficult task [7, 8]. Moreover, in everyday clinical practice there are cases of this disease in which the use of a number of drugs is limited, for example in the case of pregnancy [9, 10]. Currently, one of the most effective and safe drugs for the treatment of IBS is trimebutine, which from a modern point of view is considered not just as an antispasmodic, but as a means of regulating gastrointestinal motility [9]. This drug is considered an agonist of peripheral μ-, δ-, and κ-opiate receptors, acting throughout the gastrointestinal tract [9, 11]. The result is a dual effect of trimebutine on gastrointestinal motility, which depends on the predominance of inhibitory or excitatory influences. Thus, the modulation of propulsive motility of the gastrointestinal tract when using trimebutine manifests itself in a normalizing effect in both hypo- and hyperkinetic disorders of motor function [9, 12]. The antispastic effect of trimebutine is realized through the blockade of Na+ channels, as well as through the blockade of L-type Ca2+ channels [9, 13, 14]. Also, a number of experimental studies have shown a decrease in K+ currents in smooth myocytes induced by trimebutine, which may also play a role in the antispastic effect of the drug [15, 16]. The analgesic effect of trimebutine occurs by acting on the peripheral endings of sensory neurons associated with visceral afferent C-fibers [13].
Trimebutine has a significant evidence base in the treatment of IBS. According to the results of a meta-analysis of 23 randomized controlled trials (1888 patients), antispasmodics were more effective than placebo in improving the general condition of the patient, with an odds ratio (OR) of 2.13 (95% confidence interval [CI] - 1.77-2.58) [ 17]. At the same time, trimebutine turned out to be the most effective (OR = 3.45; 95% CI – 2.03–5.86) [17]. According to the recommendations of the Russian Gastroenterological Association (RGA) and the Association of Coloproctologists of Russia (AKR) for the diagnosis and treatment of IBS (2017), trimebutine is safe with long-term use, effective in the treatment of combined functional pathology (in particular, with a combination of IBS and functional dyspepsia), as well as effectively reduces the frequency and severity of abdominal pain [18].
In this article, we present selected clinical cases demonstrating the objective difficulties associated with the management of patients with IBS, as well as the effectiveness of trimebutine in these situations.
Clinical observation No. 1
Patient N., 32 years old, consulted a gastroenterologist with complaints of frequent stabbing, cutting, sometimes aching pain localized in the periumbilical and iliac region, somewhat decreasing after defecation and the passage of intestinal gases, noisy rumbling in the abdomen, and flatulence. Stools of a mushy consistency 2-3 times after meals with a significant admixture of mucus in the stool.
Anamnesis
Considers himself sick for 14 years, when for the first time aching pain in the lower abdomen, instability of stool with a tendency to diarrhea began to appear occasionally. She independently took over-the-counter medications (drotaverine, aluminum-containing antacids, sorbents) without significant improvement. Over the past period, the patient has repeatedly contacted gastroenterologists. Specialists prescribed short courses of antispasmodics (mebeverine, pinaverium bromide). While taking the above drugs, positive clinical dynamics of varying severity were noted, but after some time the symptoms recurred. Over the past 2 years, he has noted an increase in the number of episodes of abdominal pain and an increase in pain intensity. The patient underwent colonofibroscopy on 01.2018 and 12.2018; no organic pathology was identified. The real exacerbation occurred 2 weeks ago against the background of the patient’s usual diet.
Denies bad habits. Denies allergic history. Heredity is not burdened.
Objective data
The condition is satisfactory. Asthenic physique. Body mass index – 19.3 kg/m2. The skin and visible mucous membranes are of normal color and normal moisture. Auscultation of the lungs and heart revealed no pathology. The tongue is moist, coated at the root. The abdomen is distended due to intestinal gases, moderately painful in the mesogastrium and right iliac region. Liver along the edge of the costal arch. The spleen is not palpable. Unformed stools up to 3-4 times a day, mushy, in small portions with a significant amount of mucus. There are no macroscopic impurities of blood in the stool. The patient is anxious, convinced that she has a serious undiagnosed disease due to frequent recurrence of complaints.
Results of laboratory and instrumental examination methods
Complete blood count, biochemical blood test, general urine test without clinically significant findings.
Coprology: unformed stool, soft consistency, pungent odor, reaction to occult blood is negative, a small amount of digested and undigested fiber, a little soap, no leukocytes and red blood cells found, a large amount of mucus.
Stool calprotectin – 68 mcg/g (normal value is less than 50 mcg/g).
Indirect hemagglutination reaction (IRHA) with Salmonella O3AG, OAG; RNGA with shigellosis Zone AG, Flexner AG; RNGA with pseudotuberculosis hypertension is negative.
Ultrasound examination of the abdominal organs, as well as esophagogastroduodenoscopy without clinically significant findings.
Colonofibroscopy: no organic pathology was detected throughout the examined areas of the intestine.
In a general analysis of the anamnesis, clinical picture and data from laboratory and instrumental examination methods of the patient, there were no symptoms of anxiety (rectal bleeding, the presence of macro- and micro-impurities of blood in the stool, weight loss, anemia and other changes in blood tests, fever, onset of the disease at an older age). 50 years old, cancer and inflammatory bowel diseases in relatives, nocturnal symptoms). According to the recommendations of the Russian State Administration for the diagnosis and treatment of IBS, as part of a screening study, C-reactive protein, thyroid-stimulating hormone, IgA + IgG to tissue transglutaminase were determined - all indicators were within normal limits.
Diagnosis: IBS with diarrhea predominance
Therapy
Trimebutine 200 mg 3 times a day 30 minutes before meals, as well as simethicone 120 mg 3-4 times a day, bifid-containing probiotics in the recommended daily dose. With this therapy, the clinical symptoms of the disease were relieved within 2 weeks. The patient noted an improvement in overall health; the fear associated with the imperative urge to defecate disappeared. Upon completion of the course of treatment, the patient is recommended to continue taking trimebutine for the next three weeks.
Further observation of the patient and her keeping a food diary revealed a clear connection between the recurrence of abdominal pain syndrome and the psycho-emotional stress she experienced. The increase in the intensity and frequency of pain episodes over the past 2 years could be associated with the fact of a job change: the patient became the main consultant of the enterprise, which required her to make public appearances analyzing the work being done both at the enterprise and at conferences. The patient is recommended trimebutine 200 mg 3 times a day for a course of 7–10 days, starting 3 days before the planned public speech. As a result, the prophylactic administration of trimebutine that we recommended allowed us to either completely eliminate the appearance of abdominal pain syndrome or significantly reduce its duration and intensity. The patient repeatedly refused the proposed consultation with a psychotherapist.
Discussion
The considered clinical case demonstrates that IBS is characterized by an undulating course with alternating periods of remission and exacerbations, often provoked by psycho-emotional stress. Most patients deny the idea expressed by the doctor about the connection between abdominal symptoms and psycho-emotional stress and refuse to consult a psychotherapist, even if such consultation is available as part of free medical care. During the dynamic observation of a patient after a stopped exacerbation of IBS, we recommend keeping a food diary, in which the patient notes the dynamics of complaints against the background of the therapy, and after its discontinuation, if abdominal symptoms occur (bloating, pain, flatulence, etc.) analyzes the food consumed the day before , the presence of physical and psycho-emotional stress. Thus, by tracking cause-and-effect relationships, the patient himself comes to the conclusion about the greater role of psycho-emotional or dietary factors in the genesis of exacerbations of IBS. The prophylactic administration of trimebutine described above allows one to avoid severe exacerbations of the disease, often associated with loss of ability to work.
Clinical observation No. 2
Patient A, 25 years old, was referred by an obstetrician-gynecologist for a consultation with a gastroenterologist due to complaints of aching pain in the lower abdomen that occurs before defecation, rumbling, transfusion in the abdomen, and stool retention for up to 2-4 days.
Anamnesis
Considers himself sick for 3 months. The patient began to notice the need to strain, and short-term unexpressed pain appeared before defecation. Taking lactulose brought relief, but required an increase in the daily dose of the drug, and therefore, over the last month, the obstetrician prescribed polyethylene glycol, which did not bring a pronounced clinical effect. The patient began to notice increased abdominal pain before defecation, and independently additionally used microenemas and rectal suppositories.
When collecting anamnesis, it was revealed that at the age of 15 to 22 years, she often noted the occurrence of pain of varying intensity, localized in the lower abdomen or taking on a diffuse nature. As a result of the examination, incl. and inpatient, diagnosed with IBS, mixed variant. The patient was prescribed courses of antispasmodics (mebeverine, pinaverium bromide, alverine citrate with simethicone) with a pronounced positive effect. At the age of 23 to 25 years, relative well-being was noted - episodes of abdominal pain occurred extremely rarely and were of minimal severity. In 2015, at the age of 21, the patient underwent colonofibroscopy.
Denies bad habits. Denies allergic history. Heredity is not burdened.
Objective data
Pregnancy at 21 weeks. The condition is satisfactory. The skin and visible mucous membranes are of normal color and normal moisture. Auscultation of the lungs and heart revealed no pathology. The tongue is moist, coated at the root. The abdomen is enlarged due to pregnancy. On palpation, pain is noted in the right and left iliac regions. The liver and spleen are not palpable. Over the past week, stools have been 1 time every 2–4 days, requiring strong straining with a significant amount of mucus and streaks of blood.
Results of laboratory and instrumental examination methods
Complete blood count, biochemical blood test, general urine test without clinically significant findings.
Coprology: stool is formed, the consistency is hard, the smell is pungent, fecal, the reaction to occult blood is positive, a small amount of digested and undigested muscle fibers, a few fatty acid salts, no leukocytes and red blood cells were found, a large amount of mucus.
Stool calprotectin – 30 mcg/g (normal value is less than 50 mcg/g).
Ultrasound examination of the abdominal organs, as well as esophagogastroduodenoscopy without clinically significant findings.
The patient was scheduled to consult a proctologist. Conclusion: chronic hemorrhoids in the acute stage.
In a general analysis of the anamnesis, clinical picture and data from laboratory and instrumental examination methods of the patient, there were no symptoms of anxiety (rectal bleeding, the presence of macro- and micro-impurities of blood in the stool, weight loss, anemia and other changes in blood tests, fever, onset of the disease at an older age). 50 years old, cancer and inflammatory bowel diseases in relatives, nocturnal symptoms). According to the recommendations of the Russian State Administration for the diagnosis and treatment of IBS, as part of the screening study, C-reactive protein, thyroid-stimulating hormone, IgA + IgG to tissue transglutaminase were determined - all indicators were within normal limits.
Diagnosis. IBS associated with constipation.
Therapy
Trimebutine 200 mg 3 times a day 30 minutes before meals, as well as dimethicone and guaiazulene 3 g 3-4 times a day, psyllium 3.25 g 2 times a day, therapy prescribed by a proctologist. The therapy led to the relief of abdominal pain by the end of the first week of taking the drugs, to the normalization of bowel movements with stool passing once every 1–2 days, to the complete cessation of blood excretion in feces.
As part of the dynamic observation, the presence of blood in the stool was not noted against the background of normalization of stool; 1 month after the end of taking trimebutine (in the 35th week of pregnancy), pain in the lower abdomen recurred of significantly less intensity, short-lived, and therefore the use of trimebutine was resumed within 10 days with complete regression of symptoms.
Discussion
The range of medications approved for use during pregnancy is limited. Of the antispasmodics, the only drug that has no restrictions for use from the second trimester of pregnancy is trimebutine.
A feature of the case was the presence of streaks of blood in the stool of this patient, which required excluding the onset of inflammatory bowel disease (IBD). Although the current prevailing opinion is that the nature of the course of IBD during pregnancy depends on the activity of the disease at the time of conception, nevertheless, a number of authors note a high frequency of onset of IBD in pregnant women, especially in the first and second trimesters.
Colonoscopy and sigmoidoscopy in the second and third trimesters is limited due to the risk of premature contractions. The positive dynamics of clinical manifestations and hemotochesis against the background of therapy with antispasmodics and laxatives made it possible to minimize the likelihood of IBD in this patient.
The diagnosis of IBS was made on the basis of the complaints presented, the results of the examination, and anamnestic data. Analysis of social factors led to the idea of frequent exacerbations of IBS against the background of stress associated with education (graduate school, higher education), with a favorable course of the disease at the age of 23–25 years, when the patient got married and did housework.
Conclusion
The reviewed clinical observations demonstrate objective difficulties associated with pharmacotherapy of patients with IBS. In both cases, trimebutine was the optimal drug in terms of both effectiveness and safety. Thus, the use of trimebutine, which has a normokinetic effect on the motility of the lower gastrointestinal tract, as well as antispastic and analgesic effects, is considered a priority treatment strategy for patients suffering from IBS.
Niaspam
An analogue of Trimedat is Niaspam with the active ingredient - mebeverine hydrochloride (Niaspam is also an analogue of Duspatalin, Dutan, Mebeverine). Available in capsules of 200 mg of active principle. Appointed from age 18. Indicated for relieving intestinal spasms, contraindicated in case of individual intolerance to the components of the product, pregnancy, lactation. The drug can cause a skin rash, Quincke's edema and other manifestations of allergies.
In case of kidney pathology, the dosage is not adjusted.
Price – 364 rubles.
Sparex
The myotropic antispasmodic Sparex is an analogue of Trimedat with the active component in the form of mebeverine hydrochloride. The drug is available in capsules with 200 mg of active ingredient. Sparkex is a cheap domestic substitute not only for Trimedat, but also for Dutan and Mebeverine. The essence of the action is the relief of intestinal spasms without involvement in the process of correction of peristalsis. Appointed from age 12.
The drug should not be used while driving a car or operating precision equipment due to the risk of dizziness and drowsiness.
The dosage is individual: one capsule three to four times a day until the therapeutic effect occurs, then the dosage is reduced to maintenance (the dose is calculated by the doctor).
Adverse reactions when taking the drug: cephalalgia, vertigo, drowsiness, allergies, flatulence, dyspepsia, bronchospasm.
Price – 342 rubles.
Trigun
The cheapest analogue of Trimedat based on dicycloverine hydrochloride. The drug is produced in India in tablet form, then paracetamol is added to the main active ingredient, and in an ampoule: in 1 ampoule - 20 mg of the active substance. Indications for use are: pain, inflammation and associated spasm of intestinal smooth muscles. The tablets relieve headaches, relieve fever, and are used for algodismenorrhea and neuralgia.
The drug is prescribed in tablets from the age of 15. Prohibited for ulcerative stomach, hypovolemia, myasthenia gravis, during pregnancy, breastfeeding, allergies to components.
Trigan solution is recommended for relieving colic of any origin, but only from 6 years of age. The remaining contraindications are common to the tablet form.
Price – 92 rubles.
Enterosan
This is an enzymatic analogue of Trimedat (lyophilisate of the secretion of simple glands of the gastric mucosa of birds), has bacteriostatic, lipolytic, detoxifying and immunomodulatory effects, restores impaired absorption. Produced in Russia in capsules of 300 mg of active substance.
The drug is prescribed for the treatment of gastritis, enteritis, colitis, pancreatitis, dysbiosis, irritable bowel syndrome, diverticula, dermatoses, gastrointestinal infections, and stone formation in the gallbladder.
Contraindicated in case of allergies to components, in the first trimester of pregnancy or lactation.
Take the drug half an hour before meals, swallow the capsules whole with a small amount of water.
The dose is adjusted according to the severity of the pathology:
- acute phase – 600 mg three times a day for 12 days;
- chronic – 300 mg three times a day for 20 days;
- for prevention – 300 mg twice a day for a month.
The course can be repeated after a couple of months.
Side effects: allergies, dry skin and mucous membranes, erythema, dyspepsia, insomnia, chronic fatigue syndrome, vertigo.
Price – 378 rubles.
Trimebutin is a unique antispasmodic and prokinetic agent of the gastrointestinal tract: scientific dossier and clinical studies
Treatment of functional diseases of the gastrointestinal tract represents an unresolved medical problem. It has now been proven that visceral hypersensitivity plays a significant role in the development of this pathology, which in many cases is formed under the influence of chronic stress and marks the dysfunction of physiological anti-stress systems, in particular the opiateergic system. In this regard, the use of the “balancing” regulator of gastrointestinal motility, the opiate receptor agonist trimebutine, for the treatment of functional diseases seems promising. Experimental and clinical studies allow us to consider trimebutine as a targeted drug for the correction of visceral hypersensitivity in irritable bowel syndrome and functional dyspepsia. This review examines the mechanism of drug action of trimebutine and its effectiveness as a unique prokinetic and antispasmodic in functional diseases of the gastrointestinal tract.
Rice. 1. Nociceptive pathways and the site of action of opiates in pain therapy [4]
Rice. 2. Mechanisms of stress induction of visceral hypersensitivity [8]
Table 1. Normalization of gastric emptying parameters under the influence of trimebutine in patients with FD [41]
Rice. 3. Comparative effectiveness of prokinetics in the treatment of FD
Table 2. Intensity of the effect of prokinetics
Rice. 4. Comparative effectiveness of treatment regimens containing trimebutine or antispasmodics in patients with functional gastrointestinal disorders (IBS, FD, combination of IBS and FD) and the effect of these drugs on the main symptoms of functional disorders (modified
Table 3. Proportion of patients with a more than 30% reduction in pain/discomfort by day 29 from the start of treatment
Table 4. Reduction in the severity of pain/discomfort by the 29th day from the start of treatment
Rice. 5. Dynamics of changes in the total score on the Gastrointestinal Symptoms Rating Scale
Rice. 6. Dynamics of changes in symptoms of functional diseases in points on the Gastrointestinal Symptoms Rating Scale during treatment with Trimedat forte (A) and trimebutine 200 mg (B)
Trimebutine was synthesized by Laboratoires Jouveinal (France) in 1969. For almost 50 years in various countries around the world, trimebutine as an effective and safe antispasmodic has been successfully used to treat functional disorders of the gastrointestinal tract (GIT) in children and adults. During clinical use, an extensive scientific database has been accumulated, which has made it possible to clarify the mechanisms of action of trimebutine. It has been established that trimebutine has modulating prokinetic activity and has a “balancing” effect on gastrointestinal motility, that is, it is a special antispasmodic for the gastrointestinal tract [1, 2].
It has been proven that the medicinal effect of trimebutine is due to interaction with opiate receptors (OR) of the gastrointestinal tract. Trimebutine and its active metabolite (nortrimebutine) are nonspecific agonists of peripheral, predominantly κ- (enkephalinergic) and nociceptive, ORs throughout the sensory nerve pathways that conduct signals from mechanical and pain receptors of the gastrointestinal tract to the central nervous system (CNS).
In patients with functional gastrointestinal diseases, the mechanisms of physiological regulation are significantly altered. In particular, with functional dyspepsia (FD) and irritable bowel syndrome (IBS) with constipation, the rhythmic activity of the gastrointestinal tract at rest is disrupted: the frequency of the intestinal migratory motor complex (MMC) and the formation of propulsive peristalsis are reduced. At the same time, in patients with functional gastrointestinal diseases, the tolerance of the enteric nervous system to stretching and the perception of pain associated with excessive stretching (the phenomenon of visceral hypersensitivity) is sharply reduced. Hypersensitivity to stretch and environmental stress has been shown to be a major cause of abdominal pain in IBS [3].
The formation of visceral hypersensitivity in functional diseases of the gastrointestinal tract is regarded by most researchers as the result of stress-induced neuromodulation of the brain-gastrointestinal axis - sensitization of the enteric and central nervous systems under the influence of chronic psychological and emotional stress that these patients experience from childhood. It is known that during short-term stress and in patients without visceral hypersensitivity, stress-induced neuromodulation of the brain-gastrointestinal axis is opposed by the so-called stress-limiting systems. In the gastrointestinal tract, stress-limiting systems include a number of main neurotransmitters - acetylcholine antagonists: serotonin, norepinephrine, dopamine and endogenous opiate peptides. These amines can act both centrally and peripherally to mediate the effects of the sympathetic system.
By suppressing the activity of cholinergic neurons, stress-limiting systems reduce secretion, mobility and relax sphincters, increase the pain threshold in the brain-gastrointestinal tract axis (Fig. 1). However, long-term exposure to chronic stress leads to persistent activation of the hypothalamic-pituitary axis against the background of a gradually developing relative deficiency of stress-limiting systems.
In patients with functional gastrointestinal diseases, chronic stress causes stereotypic neuromodulation in the brain-gastrointestinal axis, aimed at reducing tolerance to pain perception and the formation of visceral hypersensitivity (Fig. 2) [5–8]. According to clinical studies, impaired perception of normal stimuli from the intestines is recorded in at least 60% of patients with IBS [7].
Thus, the analgesic and antispasmodic effect of trimebutine in IBS is based on the inhibition of impulses from the nerve endings of the enteric nervous system in the intestinal wall to the sensory neurons of the dorsal ganglia of the spinal cord. Trimebutine, like other opiate peptides, calcium channel blockers and GABAergic drugs, norepinephrine or serotonin reuptake inhibitors, reduces the perception of pain by interrupting the conduction of the pain impulse at the level of primary afferents, second order neurons or interneurons of the spinal cord (see Fig. 1) [9, 10]. The severity of blockade of pain impulses during therapy with trimebutine is comparable to that of local anesthetics such as lidocaine [10].
In contrast to the “classical” agonists of peripheral ORs, in clinical studies, trimebutine proved to be most effective in the treatment of abdominal pain and constipation in patients with functional gastrointestinal diseases [14, 15].
One of the first clinical studies using trimebutine by K. Lüttecke showed that the drug's effects were focused on stress-induced motor impairment and abdominal pain in patients with IBS [16, 17]. Somewhat later, M. Galeone et al. (1986) found that in patients with IBS, taking 600–800 mg of trimebutine orally increases propulsive peristalsis of the colon, neutralizes spontaneous and vibrating contractions, and generally normalizes colon motility [18]. In another study from the same period, S. Shannon et al. (1989) showed that oral administration of even 200 mg of trimebutine is able to synchronize postprandial motor activity of the sigmoid colon in patients with IBS with constipation, without affecting gastrointestinal motility in healthy volunteers [19]. JC Shang et al. (1993) also showed that, by reducing intestinal transit time from 105 ± 9 to 60 ± 11 hours in patients with IBS, trimebutine does not affect normal fasting and postprandial gastrointestinal motility [20].
The effect of trimebutine on IBS symptoms in randomized clinical trials (RCTs) was first assessed in a meta-analysis by T. Poynard et al. [21]. A meta-analysis of 26 RCTs was the first to show the greater efficacy and safety of trimebutine in correcting IBS symptoms compared to other antispasmodics and placebo. The targeting of trimebutine against IBS symptoms caused by visceral hypersensitivity was shown in a systematic review by M. Delvaux and D. Wingate [1]. Based on an analysis of experimental data and 12 RCTs, the authors demonstrated that trimebutine effectively relieves the pain syndrome characteristic of IBS and increases the threshold of pain sensitivity, reducing visceral hypersensitivity and positively modifying the subjective perception of visceral pain. Later, a number of studies also showed that, simultaneously with the correction of IBS symptoms, trimebutine has a positive effect on the psychological status of patients, reducing their anxiety and negative involvement in their disease [22].
In 2008, the effectiveness of trimebutine in the treatment of functional abdominal pain and dyspepsia was confirmed in a meta-analysis of 22 RCTs on the use of antispasmodics with different mechanisms of action, including 1778 patients with IBS [23]. In patients with IBS, the effectiveness of antispasmodics as a class of drugs was 53–61% and was higher than placebo (31–41%). The NNT indicator (the number of patients who need to be treated to achieve a positive result in one patient) when using antispasmodics varied from 3.5 to 9.
In 2009, the effectiveness of trimebutine among other antispasmodics with different mechanisms of action (hyoscine, hyoscyamine, otilonium bromide, pinaverium bromide, alverine, mebeverine, pirencipine and some others not registered in Russia) was analyzed by a working group on the study of IBS [24]. The systematic review included 22 studies involving 1778 patients. According to experts, all antispasmodics, including trimebutine, despite some inevitable methodological defects in studies when studying functional diseases, were more effective in the treatment of IBS than placebo (odds ratio (OR) 0.68, 95% confidence interval (CI) 0. 57–0.81). In 2011, trimebutine was included in a Cochrane systematic review of drugs of various types of action used to treat IBS. According to L. Ruepert et al., trimebutine is an effective antispasmodic for the relief of abdominal pain in patients with IBS [25].
L. Rurpert et al. [25] analyzed the effect of therapy on the severity of abdominal pain in IBS (56 RCTs, 3725 patients). They compared the effectiveness of placebo, plant fiber and psyllium (12 studies), four antispasmodics (29 studies) - dicyclomine, peppermint oil, pinaverium, trimebutine, two antidepressants (15 studies) - serotonin reuptake inhibitors and amitriptyline. The researchers concluded that plant fiber does not affect the severity of abdominal pain in IBS. Antispasmodics are effective in patients with abdominal pain due to IBS. Serotonin reuptake inhibitors and tricyclic antidepressants are also effective, but the effect depends on individual patient characteristics.
In 2013 and 2015, respectively. The effectiveness of trimebutine continued to be studied in comparison with the most widely used antispasmodics for the treatment of IBS: pinaverium (GS Karabulut et al., 2013) and mebeverine (MZ Rahman et al., 2015). According to both RCTs, trimebutine was no less effective than pinaveria bromide and mebeverine in reducing abdominal pain and increasing the frequency of bowel movements in patients with IBS [26, 27]. In 2015, these data were confirmed in a review by the Canadian Agency for Drugs and Technologies in Health, which also showed that trimebutine was more effective than both of these antispasmodics in improving the quality of life of patients with IBS, especially in the pediatric population [28]. At the same time, therapy with trimebutine was safer - it was devoid of adverse effects characteristic of other classes of antispasmodics: muscle relaxants, anticholinergics and pinaverium bromide
.
The tendency of these agents to self-induce constipation, frequent dizziness, dry mouth, paralysis of accommodation, urinary retention, and possible confusion in elderly patients limits their usefulness in clinical practice [29]. Thus, the level of evidence of studies that confirmed the effectiveness of trimebutine corresponded to category I, the level of practical recommendations corresponded to category A
[30].
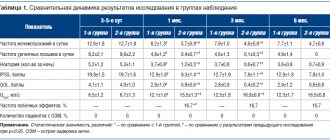
Many studies have shown the effect of trimebutine on upper gastrointestinal motility and its effectiveness as an antispasmodic and prokinetic in the treatment of FD. In the already mentioned “early” systematic review, M. Delvaux and D. Wingate (1997) showed that in patients with FD, a course of trimebutine effectively reduces the total time food remains in the stomach [1]. According to A. Aktas (1999), this happens because in PD, trimebutine significantly reduces the lag period (VLAG) - the time from the beginning of food entering the stomach until the beginning of its evacuation and reduces the volume of food retained in the stomach (R100 - percentage of food received volume of food remaining in the stomach after 100 minutes) (Table 1) [31].
Thus, trimebutine restores both the normal time after which the stomach should begin to evacuate food and the normal duration of this process by inducing phase III MMC in the small intestine. This effect is not directly related to hyperacidity in the stomach and is fully preserved during treatment with proton pump inhibitors [32]. According to some data, trimebutine can no less effectively stimulate the motility of the gastric outlet even in comorbid and seriously ill elderly patients with gastroparesis due to cerebrovascular pathology and a reduced level of consciousness, as well as contribute to the resolution of postoperative paresis of the stomach and intestines, gastroparesis in insulin-dependent diabetes mellitus [33– 35].
Meta-analysis by T. Hiyama et al. (2007), dedicated to assessing the comparative effectiveness of prokinetics as the main class of drugs for the treatment of FD, included 27 placebo-controlled RCTs using trimebutine and other prokinetics (1844 patients received treatment, 1591 received placebo). A meta-analysis demonstrated the effectiveness of even short-term (seven days at a dose of 600 mg) administration of trimebutine for the correction of FD symptoms [36].
In a recent meta-analysis by YJ Yang et al. (2017) also compared the effectiveness of various prokinetic agents in the treatment of FD (25 RCTs, 4473 patients). When prescribed as a course, trimebutine turned out to be second after metoclopramide in terms of integral effectiveness against FD symptoms [37]. The therapeutic effectiveness of trimebutine was almost the same as that of metoclopramide (OR 1.32, 95% CI 0.27–6.06) and was higher than the effectiveness of mosapride (OR 1.99, 95% CI 0.87–4.72), domperidone (OR 2.04, 95% CI 0.92–4.60) and itopride (OR 2.79, 95% CI 1.29–6.21) (Fig. 3, Table 2).
According to the authors' conclusions, given the risk of adverse events during therapy with metoclopramide or domperidone, patients with FD are recommended to be prescribed short courses of these drugs or a safe alternative - therapy with trimebutine or mosapride.
Most RCTs using trimebutine emphasize the greatest effectiveness of treatment in patients with initially more severe symptoms and overlapping symptoms of functional gastrointestinal diseases. Thus, in a population of patients with a combination of FD and IBS, as well as a high frequency of diarrheal syndrome, the administration of trimebutine led to the normalization of colonic motility, acceleration of propulsive peristalsis and colonic transit (60 versus 95 and 105 hours, respectively) due to the activation of electrophysiological activity of the intestine [38] .
In Russia, the effectiveness of trimebutine in patients with FD, IBS and patients with a combination of FD and IBS was confirmed in an observational study (254 patients, 30 with IBS, 67 with FD, 157 patients with a combination of IBS and FD) [40]. Its goal was to study practical algorithms for pharmacological treatment and the comparative effectiveness of motility regulators and probiotics in patients with functional gastrointestinal diseases (trimebutine in treatment regimens was used in a standard dose during a course of administration). Trimebutine was significantly more effective in reducing the severity of each symptom of functional disorder compared to regimens that included antispasmodics, with the exception of constipation and abnormal stool consistency (hard stool), for these symptoms the effectiveness of trimebutine seemed comparable to that of antispasmodics (Fig. 4).
Most RCTs using trimebutine (with the exception of studies in intensive care unit patients) assessed the effectiveness and safety of a course of the drug (two to four weeks). Comparing the effects of course and short-term (one to three days) treatment with trimebutine, the authors unanimously note that the positive properties of the drug are most evident not in the first days, but after two or more weeks of treatment [39]. Taking into account the active first-pass metabolism of trimebutine, it was suggested that with very good tolerability of the drug, increasing the single dose of trimebutine to 300 mg while maintaining the daily dose of 600 mg could lead to an increase in the effectiveness of treatment while improving patient compliance, since the drug intake will be reduced to two times per day. day.
A study of the effectiveness and safety of trimebutine 300 mg, one tablet twice a day, for a total of 600 mg per day (Trimedat® forte, extended-release film-coated tablets, Valenta Pharm JSC, Russia) during a course (28 days) treatment of pain , caused by functional diseases of the gastrointestinal tract and/or biliary tract, was performed in an open, multicenter, prospective, randomized, comparative study of the effectiveness and safety of phase III [40]. The effectiveness and safety of Trimedat® forte was compared with trimebutine 200 mg produced in France, one tablet three times a day, for a total of 600 mg per day. The study was conducted in 2015–2016. on the bases of 16 large gastroenterological clinics in Moscow, St. Petersburg, Yaroslavl, Ryazan, Nizhny Novgorod, Ufa, Chelyabinsk, Pyatigorsk and Reutov. The study included patients with chronic abdominal pain in combination with at least one of the symptoms of dyspepsia - heartburn, belching, nausea, flatulence, constipation, diarrhea and a verified diagnosis of one of the functional gastrointestinal diseases (non-erosive gastroesophageal reflux disease, functional nausea/vomiting, FD, IBS, biliary dysfunction). The patients included 56 men (27%) and 151 women (73%) aged 43.3 ± 13.6 years (range 18 to 69 years). The effectiveness of treatment was assessed by the frequency of a positive response to treatment - the proportion of patients in the group with a decrease in complaints by more than 30% on the Gastrointestinal Symptoms Rating Scale by the 29th day from the start of treatment (primary endpoint) and the dynamics of changes in the total score on the Scale were determined assessment of gastrointestinal symptoms by 7, 14, 21 and 29 days from the start of treatment, dynamics during treatment of Gastrointestinal Symptoms Rating Scale scores for individual syndromes, dynamics of changes in quality of life according to the SF-36 questionnaire by 29 days from start of treatment (secondary end points) (Table 3, 4, Fig. 5, 6).
Quality of life, assessed by the physical and psychological components of the SF-36 questionnaire, improved by an average of 1.2 times by the 29th day from the start of treatment compared to the initial state and also demonstrated non-inferior effectiveness of Trimedat® forte compared to trimebutine 200 mg produced in France according to this criterion.
An assessment of the effect of food intake on the pharmacokinetics of the drug, carried out in some patients in a phase III clinical trial, showed that taking the drug simultaneously with a high-calorie meal increases the bioavailability of nortrimebutine by 25.9% (median AUC0-t ratio) compared with administration on an empty stomach.
Data from a clinical study confirmed the high effectiveness of the new dosage form of the drug Trimedat® forte in relieving abdominal pain and manifestations of dyspeptic syndrome. Reducing the frequency of drug administration led to an increase in patient adherence to the prescribed treatment.
Thus, providing prokinetic, antispasmodic and analgesic effects, trimebutine occupies a special place in the limited arsenal of drugs for the treatment of functional gastrointestinal diseases. The point of application of trimebutine as a peripheral OR agonist is the multi-level afferent nervous structures of the gastrointestinal tract. The interaction of trimebutine with the gastrointestinal tract leads to:
- to inhibition of acetylcholine release from enteric plexus neurons and neuromodulation of acetylcholine activity in relation to gastrointestinal smooth muscles;
- release of the effects of endogenous anticholinergic stress-limiting systems of the gastrointestinal tract;
- blocking the transmission of painful and non-painful stimuli in NMDA synapses of neurons of the posterior spinal ganglia to interneurons, which reduces the transmission of pathological stimuli to the central nervous system with a subsequent decrease in the effector response;
- increasing tolerance to painful and mechanical stimuli – leveling visceral hypersensitivity.
In medical practice, the relationship between central and peripheral effects in the medicinal action of trimebutine is often understood in a simplified manner. In the mechanism of action of trimebutine, a significant role is played by the interaction with sensitive nervous structures, which have a significantly higher concentration of OP than the wall of the gastrointestinal tract. According to experimental studies, in the wall of the small intestine the content of nociceptive ORs (δ-ORs) is 15–20 times less than in the dorsal ganglia of the spinal cord, and 100 times less than in the brain [41]. It is the breadth of trimebutine’s effect on multi-level ORs located “below” the brain (the drug does not penetrate the blood-brain barrier) that makes it possible to provide a stable antispasmodic and analgesic effect in functional gastrointestinal diseases mediated by CNS pathology and the formation of visceral hypersensitivity.
According to numerous RCTs, trimebutine has a modulating effect on the gastrointestinal tract, which is indispensable in the treatment of diseases, the main pathogenesis of which is visceral hypersensitivity and high smooth muscle tone in patients with stress-induced pathology: FD, IBS with constipation and a mixed version of IBS, chronic idiopathic constipation . The modulating effect of trimebutine on the motility and muscle tone of the gastrointestinal tract - correction of impaired intestinal motility by inducing the regulation of spontaneous activity without affecting normal motility - is due to varying degrees of stress-induced neuromodulation of the brain-gastrointestinal tract axis, and, consequently, sensitization of the OR in different patients with functional diseases. A number of clinical studies have convincingly shown that the effect of trimebutine, at least the nature of the effect on gastrointestinal motility, depends on the vector of its previous disturbance. Thus, the effect of trimebutine on abdominal pain characteristic of IBS due to intestinal distension against the background of hypersensitivity will be more pronounced the higher the sensitivity of the nervous system to pain and the lower its tolerance to any peripheral proprioceptive impulses that normally should not be recognized by the central nervous system. According to some authors, trimebutine is effective in IBS with constipation because in patients with visceral hypersensitivity there is an adaptive hyperreactivity of peripheral opiatergic structures, sensitization and secretion of new ORs. For example, it has been shown that in the intestines of mice, experimental inflammation and bacterial overgrowth stimulate the secretion of new ones and increase the sensitivity of μ-ORs [42]. Clinically, this leads to the development of gastrointestinal dysfunction characteristic of an overdose of exogenous opiate analgesics: nausea/vomiting, anorexia, constipation, a feeling of incomplete emptying, bloating, abdominal discomfort, gastroesophageal reflux, intestinal pseudo-obstruction [9, 10, 43]. Moreover, it is believed that all of these effects are realized through the activation of both central and peripheral μ-ORs, since they are almost completely eliminated by the non-selective OR antagonist naloxone [44]. In such patients, trimebutine (a non-selective peripheral OR agonist) exhibits the effects of a competitive OR antagonist rather than an agonist, minimizing these symptoms. On the contrary, trimebutine accelerates gastric emptying, induces premature phase III urinary tract and modulates colonic contractility. Due to the harmonization of the functions of individual ORs, trimebutine has a modulating (stimulating or relaxing) effect on the tone of smooth muscle cells and the peristaltic activity of the gastrointestinal tract, depending on their initial state. Trimebutine weakens reflexes caused by dilatation of the intestinal lumen, and thus may modulate visceral sensitivity and have an antinociceptive effect in patients with IBS [45]. The hypothesis that peripheral OR antagonists may regulate spasm and constipation due to increased expression of OR and/or increased activity of the opioidergic GI system, both under chronic stress and during long-term use of opiate analgesics, stimulates the search for new opioidergic molecules [43, 46]. Studies of the new selective peripheral gastrointestinal κ-OR antagonist fedotozine also demonstrated prokinetic properties and efficacy in the treatment of patients with functional gastrointestinal diseases [47]. However, unlike trimebutine, most of the selective agonists of the gastrointestinal tract known today (loperamide and new drugs - rakecadotril, eluxadolil, asimadoline) demonstrate the opposite effect on trimebutine on motility and are used for the treatment of acute and chronic diarrhea. In IBS with diarrhea, selective agonists reduce gastrointestinal motility and secretion, do not block visceral hypersensitivity, and have virtually no effect on abdominal pain associated with intestinal distension [29, 44, 48]. In general, against the backdrop of somewhat disappointing results of clinical studies using new selective peripheral OR agonists and highly selective modulators of different classes of serotonin receptors, the prokinetic, antispasmodic and analgesic therapeutic effect of trimebutine (Trimedat) is unique in terms of “efficacy/safety” and can be recommended as a treatment for the former series for abdominal pain, dyspepsia and constipation in patients with functional gastrointestinal diseases [49, 50].