Reaferon
Active substance: interferon alfa-2b (interferon alfa-2b)
USAN accepted for use in the USA
Dosage form
| REAFERON-EC | liof. d/prep. injection solution and local approx. 500 thousand IU: amp. 5 or 10 pcs., fl. 5 pieces. reg. No.: P N000642/01 dated 07/22/08 - Valid |
Release form, composition and packaging
Lyophilisate for the preparation of a solution for injection and local use in the form of a powder or porous mass, white, hygroscopic; when diluted, a colorless transparent or slightly opalescent solution is formed.
| 1 amp. | |
| interferon alpha-2b human recombinant | 500,000 IU |
Excipients: albumin, solution for infusion 10% - 4.5 mg, sodium chloride - 9.07 mg, sodium hydrogen phosphate dodecahydrate - 2.74 mg, sodium dihydrogen phosphate dihydrate - 0.37 mg.
500,000 IU - ampoules (5) - contour blister packs (1) - cardboard packs. 500,000 IU - ampoules (5) - contour blister packs (2) - cardboard packs. 500,000 IU - bottles (5) - contour cell packaging (1) - cardboard packs.
Indications
For adults as part of complex therapy:
- acute viral hepatitis B - moderate and severe forms at the beginning of the icteric period until the 5th day of jaundice (at a later date, the administration of the drug is less effective; the drug is not effective in developing hepatic coma and cholestatic course of the disease);
- acute protracted hepatitis B and C, chronic active hepatitis B and C, chronic hepatitis B with the delta agent, without signs of cirrhosis and with signs of liver cirrhosis;
— stage IV kidney cancer, hairy cell leukemia, malignant skin lymphomas (mycosis fungoides, primary reticulosis, reticulosarcomatosis), Kaposi's sarcoma, basal cell and squamous cell skin cancer, keratoacanthoma, chronic myeloid leukemia. Langerhans cell histiocytosis, subleukemic myelosis, essential thrombocythemia;
- viral conjunctivitis, keratoconjunctivitis, keratitis, keratoiridocyclitis. keratouveitis;
In complex therapy for children over 1 year of age:
- for acute lymphoblastic leukemia in the period of remission after the end of induction chemotherapy (at 4-5 months of remission);
- for respiratory papillomatosis of the larynx, starting the next day after removal of papillomas.
Side effect
The frequency of adverse reactions is given in accordance with the WHO classification: “very common” - 1/10, “common” - more than 1/100, but less than 1/10, “infrequent” - more than 1/1000, but less than 1/100. “rare” - more than 1/10000, but less than 1/1000 and “very rare” with an occurrence of less than 1/10000, including individual reports.
When using Reaferon-EC (in clinical studies and outside clinical studies), the following adverse events were observed:
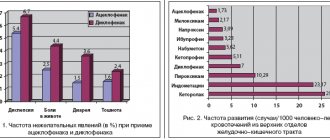
Often, with parenteral administration of the drug, a flu-like syndrome is observed (chills, fever, asthenia, fatigue, fatigue, myalgia, arthralgia, headaches), partially relieved by paracetamol and indomethacin. As a rule, a flu-like syndrome appears at the beginning of treatment and decreases with continuation.
From the cardiovascular system: rarely - arrhythmias, transient reversible cardiomyopathy; very rarely - arterial hypotension, myocardial infarction.
From the digestive system: rarely - dry mouth, nausea, abdominal pain, dyspepsia, appetite disturbances, weight loss, vomiting, diarrhea; very rarely - pancreatitis, hepatotoxicity.
From the side of the central nervous system: rarely - irritability, nervousness, depression, asthenia, insomnia, anxiety, impaired ability to concentrate, suicidal thoughts, aggressiveness; very rarely - neuropathy, psychosis.
From the skin: rarely - skin rashes and itching. increased sweating, hair loss. When injected into or under the lesion, there is rarely a local inflammatory reaction. These side effects are usually not an obstacle to continuing use of the drug.
From the endocrine system: against the background of long-term use of the drug, changes in the thyroid gland are possible. Very rarely - diabetes mellitus.
From the laboratory parameters: when using the drug, deviations from the norm in laboratory parameters are possible, manifested by leukopenia, lymphopeia, thrombocytopenia, anemia, increased activity of alanine aminotransferase, alkaline phosphatase, creatinine concentration, urea. In general, these changes are usually minor, asymptomatic and reversible.
From the musculoskeletal system: rarely - rhabdomyolysis, leg cramps, back pain , myositis, myalgia.
From the respiratory system: rarely - pharyngitis, cough, dyspnea, pneumonia.
From the urinary system: very rarely - renal failure.
From the immune system: rarely - autoimmune pathology (vasculitis, rheumatoid arthritis, lupus-like syndrome); very rarely - sarcoidosis, agioneurotic allergic edema, anaphylaxis, facial edema.
On the part of the visual organs: when the drug is applied topically on the mucous membrane of the eye, hyperemia, single follicles, and swelling of the conjunctiva of the lower fornix are possible. Rarely - retinal hemorrhages, focal changes in the fundus, thrombosis of the retinal arteries and veins, decreased visual acuity, optic neuritis, papilledema.
From the hearing organs : rarely - hearing loss.
In case of pronounced local and general adverse reactions, administration of the drug should be discontinued.
Contraindications for use
- hypersensitivity to the components of the drug;
- severe forms of allergic diseases;
- severe diseases of the cardiovascular system: heart failure in the stage of decompensation, recent myocardial infarction, severe heart rhythm disturbances;
- severe renal and/or liver failure, including those caused by the presence of metastases, chronic hepatitis with uncompensated liver cirrhosis, autoimmune hepatitis;
- epilepsy and other central nervous system dysfunctions, mental illnesses and disorders in children and adolescents;
- history of autoimmune disease;
- use of immunosuppressants after transplantation;
- diseases of the thyroid gland that cannot be controlled by generally accepted therapeutic methods;
- CC below 50 ml/min (when prescribed in combination with ribavirin), when used in combination with ribavirin, the contraindications specified in the instructions for use of ribavirin should also be taken into account;
- use in men whose partners are pregnant;
- pregnancy and breastfeeding.
Carefully
- renal and/or liver failure, severe myelosuppression.
- mental disorders, especially expressed by depression, suicidal thoughts and attempts in the anamnesis.
- patients with psoriasis, sarcoidosis.
Use during pregnancy and breastfeeding
The drug is contraindicated for use during pregnancy and breastfeeding.
special instructions
To promptly identify abnormal laboratory parameters that may arise during therapy, general clinical blood tests must be repeated every 2 weeks, and biochemical tests - every 4 weeks.
When the platelet count decreases to a value less than 50?109/l. absolute number of neutrophils less than 0.75?109/l, it is recommended to temporarily reduce the dose by 2 times and repeat the analysis after 1-2 weeks. If changes persist, it is recommended to stop treatment.
If the platelet count decreases to a value less than 25×109/l, the absolute number of neutrophils is less than 0.50×109/l, it is recommended to stop treatment.
In case of development of immediate hypersensitivity reactions (urticaria, angioedema, bronchospasm, anaphylaxis), the drug is discontinued and appropriate drug therapy is immediately prescribed. Transient skin rash does not require discontinuation of therapy.
If signs of liver dysfunction appear, the patient should be closely monitored. If symptoms progress, administration of the drug should be discontinued.
With mild to moderate renal impairment, their functional state must be carefully monitored.
Prescribe with caution to patients with severe chronic diseases, such as COPD, diabetes mellitus with a tendency to ketoacidosis, in patients with bleeding disorders, severe myelosuppression. In patients receiving Reaferon-EC for a long time, pneumopitis and pneumonia are observed in rare cases. Timely withdrawal of interferon alpha and administration of glucocorticosteroid therapy help relieve pulmonary syndromes.
In patients with thyroid diseases, before starting treatment, it is necessary to determine the concentration of thyroid hormone; it is recommended to monitor its level at least once every 6 months. If dysfunction of the thyroid gland occurs or the course of existing diseases worsens that are not amenable to adequate drug correction, it is necessary to discontinue the drug.
In case of changes in the mental sphere and/or central nervous system, including the development of depression, observation by a psychiatrist is recommended during the treatment period, as well as for 6 months after its completion. These disorders are usually quickly reversible after cessation of therapy, but in some cases it may take up to 3 weeks for them to completely reverse. If the symptoms of a mental disorder do not regress or worsen, suicidal thoughts or aggressive behavior directed at other people appear, it is recommended to stop treatment with Reaferon-EC and consult a psychiatrist. Suicidal thoughts and attempts are more often observed in pediatric patients, primarily adolescents (2.4%), than in adults (1%). If therapy with interferon alfa-2b is considered necessary in adult patients with serious mental disorders (including a history), it should only be initiated if appropriate individual screening and treatment for the mental disorder is provided. The use of interferon alfa-2b in children and adolescents with serious mental disorders (including a history) is contraindicated.
With long-term use, usually after several months of treatment, visual disturbances are possible. An ophthalmological examination is recommended before starting therapy. If you complain of any ophthalmological disorders, immediate consultation with an ophthalmologist is necessary. Patients with diseases that may cause changes in the retina, such as diabetes mellitus or arterial hypertension, should undergo an ophthalmological examination at least once every 6 months. If visual disturbances appear or worsen, discontinuation of Reaferon-EC therapy should be considered.
In elderly patients receiving the drug in high doses, disturbances of consciousness, coma, convulsions, and encephalopathy are possible. If such disorders develop and dose reduction is ineffective, treatment should be discontinued.
Patients with cardiovascular disease and/or advanced cancer require careful monitoring and ECG monitoring. If arterial hypertension develops, it is recommended to ensure adequate hydration and appropriate therapy.
In patients undergoing transplantation (eg, kidney or bone marrow), drug immunosuppression may be less effective because Interferon has a stimulating effect on the immune system.
With long-term use, interferon alfa may cause the development of interferon antibodies in some individuals. As a rule, antibody titers are low, and their appearance does not lead to a decrease in the effectiveness of treatment.
Prescribe with caution to patients with a predisposition to autoimmune diseases. If symptoms of an autoimmune disease appear, a thorough evaluation should be performed and the possibility of continuing interferon therapy should be assessed. Rarely, interferon alpha therapy is associated with the occurrence or exacerbation of psoriasis and sarcoidosis.
Impact on the ability to drive vehicles and machinery
During the period of use of the drug, patients experiencing fatigue, drowsiness or disorientation should refrain from engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Conditions for dispensing from pharmacies
The drug is available with a prescription.
Storage conditions and periods
The drug should be stored in a place protected from light and out of reach of children at a temperature not exceeding 8°C. Shelf life: 3 years.
A drug that has expired cannot be used.