Saccharomyces boulardii in gastroenterological practice
In recent years, there has been a growing number of publications devoted to various aspects of the use of probiotics not only in gastroenterology. Interest in probiotics is due to the need to use drugs with physiological effects that do not cause harm to the human body. The use of ever new antibiotics, immunosuppressants, other chemotherapeutic drugs, and hormonally active agents inevitably leads to disruption of the human intestinal microbiocenosis and weakening of immune defense mechanisms. In these conditions, the use of drugs that are alternative to antibiotics or reduce the incidence of side effects of aggressive chemotherapy is very important.
Among probiotics, the drug Enterol®, which is a non-pathogenic yeast fungus Saccharomyces boulardii obtained from tropical plants, is increasingly being used. S. boulardii lives and reproduces at an unusually high temperature of 37 °C, which corresponds to the temperature regime in the intestinal cavity (McFarl, 1995), and has a very high viability [1].
S. boulardii belongs to the ascomycetes that have genetically determined resistance to almost all groups of antibiotics, sulfonamides and other antimicrobial agents [2–5]. This property fundamentally distinguishes Enterol® from other microbial probiotics based on bacteria of obligate intestinal microflora (containing bifidobacteria, lactobacilli, fecal enterococcus) and allows its use simultaneously with a course of antibacterial therapy. S. boulardii does not suppress the growth of obligate microorganisms in the intestinal cavity.
The relationship of S. boulardii with antifungal drugs varies. Thus, fungicides that are not absorbed from the intestine (nystatin) completely suppress the growth of the fungus, and when taking absorbable fungicides (fluconazole), the viability of the yeast is completely preserved [6].
S. boulardii is resistant to hydrochloric acid and, when taken daily, is found in all parts of the gastrointestinal tract (GIT) [4, 7–9]. This yeast is a transient flora for humans, therefore, 2–5 days after the end of taking the drug, it is completely eliminated from the body without side effects [7, 10]. Enterol® is a locally acting intestinal antiseptic and is not absorbed from the gastrointestinal tract. It is mainly used for diarrhea of various origins with a predominantly secretory mechanism of development - bacterial and viral diarrhea.
The mechanism of action of Enterol® is determined by several aspects [11, 12]:
- direct antimicrobial effect;
- direct and indirect antitoxic effect (binding of microbial toxins in the intestine);
- antisecretory effect (reduces intestinal secretion of water and electrolytes during secretory diarrhea);
- direct and indirect antiviral effect;
- trophic effect (stimulates the enzymatic activity of intestinal disaccharidases and other digestive enzymes);
- nonspecific immunomodulatory effect.
Experimental data
The direct antimicrobial effect of Enterol® has been established in vitro and in vivo against a number of pathogens of intestinal infections, opportunistic microorganisms and protozoa [11, 13–16]: Salmonella typhimurium, Yersinia enterocolitica, Escherichia coli, Clostridium difficile, Shigella dysenteriae, Entamoeba histolitica , Giardia lamblia, Candida albicans, C. krusei, C. pseudotropicalis, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, etc.
Such a wide range of antimicrobial effects determines the use of Enterol® as an antidiarrheal and antiseptic agent for acute intestinal infections (as an alternative to antibiotics when their use is impossible); antibiotic-associated diarrhea and pseudomembranous colitis caused by C. difficile; parasitic diarrhea and intestinal dysbiosis, including microbial contamination of the small intestine with conditional pathogens.
The ability of Enterol® to suppress the growth of fungi of the genus Candida can be successfully used in case of fungal contamination of the intestine. In an experiment on mice in a state of artificial immunosuppression with antibiotics and prednisolone, it was shown that S. boulardii prevents the generalization of C. albicans fungi into the blood, lymph nodes and spleen [13].
The effectiveness of S. boulardii in dysentery with positive changes at the histological level has been demonstrated in mice. When using S. boulardii, the survival rate of mice was 75% versus 32% in the control group [13]. The same effect was demonstrated for S. typhimurium (50% and 20%). Interestingly, all the positive dynamics and survival of experimental animals when taking S. boulardii are not due to a decrease in the number of pathogenic microorganisms in the intestines of animals. The proposed mechanism of action is adhesion and lectin-mediated binding of infectious agents to specific sites on the outer membrane of yeast cells. This mechanism has been proven for S. typhimurium, S. enteritidis and some serotypes of E. coli [11, 15].
The antitoxic effect of Enterol®, its ability to bind bacterial cyto- and enterotoxins enhance the antimicrobial effect, and as an independent mechanism it can be used for foodborne toxic infections, for example, staphylococcal infections. Inhibition of the activity of bacterial toxins, in particular cholera and heat-labile toxin Escherichia coli, leads to a decrease in the secretion of water and electrolytes and a weakening of the diarrheal syndrome caused by these toxins [17–19]. Diarrhea associated with these infections is a classic example of cAMP-dependent secretory diarrhea. The mechanism of the antitoxic action of S. boulardii is due to the production of certain proteins.
One of these proteins, with a molecular weight of 120 kDa, does not have proteolytic activity, but inhibits the synthesis of cyclic adenosine monophosphate (cAMP) and thus reduces the symptoms of diarrhea. Thanks to this mechanism, the survival rate of animals infected with these microorganisms and treated with S. boulardii increases [20]. The other protein is a small protease (54 kDa) capable of lysing C. difficile toxin A, which stimulates intestinal secretions and increases the permeability of the intestinal mucosa. The use of S. boulardii in rats infected with clostridium helps reduce the severity of damage to the mucous membrane [5, 19, 21].
The trophic immunomodulatory effect of Enterol® is due to its ability to synthesize polyamines (spermine, spermidine) [12]. Polyamines stimulate the activity of digestive enzymes in the small intestine: they increase the production of saccharidases (lactase, maltase, sucrase) and amnopeptidases, which leads to weight gain in experimental animals [11]. The trophic effect of Enterol® is extremely important when bacteria and viruses damage the intestinal mucosa and, as a result, reduce the activity of digestive enzymes. In addition, polyamines stimulate the synthesis of IgA and components of other immunoglobulins in the intestinal mucosa [12].
The indirect antiviral activity of Enterol® is associated with its ability to increase local immune defense of the intestine, enhance the synthesis of IgA, produce polyamines and stimulate trophic effects.
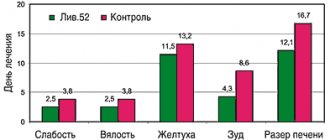
Clinical studies of the effectiveness of S. boulardii, conducted in the period 1976–2000, showed that the drug is highly effective against diarrhea of various origins, mainly of infectious origin.
Antibiotic-associated diarrhea
Double-blind, randomized studies conducted in a variety of antibiotic-requiring infections have demonstrated that the use of S. boulardii reduces the incidence of antibiotic-associated diarrhea by two to four times. Thus, in one study involving 388 patients, the incidence of diarrhea in the group of patients taking S. boulardii along with antibiotics was 4.5% versus 17.5% in the placebo group. In two other studies (180 and 193 patients), these values were 9.5% versus 22% and 7.2% versus 14.6%, respectively [22–24]. In these studies, the causative agent of diarrhea was not identified.
Acute diarrhea
The effectiveness of the drug was studied in controlled studies for acute diarrhea in adults and children. In 92 adult patients with acute diarrhea, administration of S. boulardii after 48 hours resulted in a significant reduction in the number of bowel movements and changes in stool consistency compared with the placebo group [27]. Similar results were demonstrated in 130 children aged three months to three years with acute diarrhea [28] and in two earlier randomized trials of similar design [29, 30].
Traveler's diarrhea
The double-blind, placebo-controlled study involved 1,231 international travelers [31]. The results showed that in the placebo group the incidence of diarrhea averaged about 43%, and in the group receiving S. boulardii for the prevention of diarrhea it averaged 31%, which was significantly lower. Interestingly, the study results varied by region. The highest effectiveness of the drug (i.e., the proportion of study participants without developing diarrhea) was noted in Africa (up to 59%) and on tropical islands (about 40%), in other regions this figure was lower. These differences can probably be explained by different pathogens causing traveler's diarrhea in different regions and their different sensitivities to the drug. The study used two doses of the drug: 250 and 500 mg per day. There were no differences in the incidence of diarrhea depending on the dose.
Conclusion
Although the mechanism of action of Enterol® still remains unclear, the results of its long-term clinical use indicate its high effectiveness and safety. Thanks to its complex antidiarrheal, antimicrobial, antiviral and antitoxic effects, Enterol® can be an additional or primary treatment for secretory infectious diarrhea in adults and children. The trophic, immunomodulatory and digestion-stimulating effect of the drug helps overcome the consequences of severe infections.
For questions regarding literature, please contact the editor.
E. L. Belousova , Doctor of Medical Sciences, Professor
MONIKA them. M. F. Vladimirsky, Moscow
Saccharomyces boulardii (Saccharomyces boulardii)
Saccharomyces boulardii
(lat.
Saccharomyces boulardii
) is a species of unicellular microscopic yeast fungi from the genus Saccharomycetes.
In lyophilized form, Saccharomyces boulardii
is used as an active substance in antidiarrheal and antimicrobial drugs.
Saccharomyces boulardii is
named after the French scientist Henri Boulard, who isolated it from some tropical fruits in Indochina after he noticed that the peel of these fruits was used by the local population to treat dyspeptic diseases.
Saccharomyces boulardii is also used as probiotics. Their distinctive quality from most other probiotic microorganisms (including bifidobacteria, lactobacilli and enterococci) is their resistance to the acidic environment of the stomach; they are not destroyed by gastric juice and, when administered orally, enter the intestines intact.
Saccharomyces boulardii does not colonize the intestine. 2–5 days after stopping taking drugs containing Saccharomycetes, they are no longer detectable in the stool. The half-life of viable Saccharomycetes in feces is approximately 6 hours. Saccharomyces Boulardii does not penetrate beyond the intestinal tube into the mesenteric lymph nodes and other organs and does not cause histological changes in the intestinal mucosa.
Saccharomyces boulardii increases the enzymatic function of the intestine by causing the activity of intestinal epithelial disaccharidases: lactase, sucrose-alpha-glucosidase and maltase. There is evidence (Buts et al., 1986) that taking Saccharomyces Boulardii increases the activity of lactase by 77%, sucrase by 82%, and maltase by 75%.
Saccharomycetes boulardii have a direct antagonistic effect against many types of pathogenic and opportunistic microorganisms: Candida krusei, Candida pseudotropicalis,
Candida albicans, Clostridium difficile, Gardia lambliae, Klebsiela spp. , Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, Salmonella typhimurium, Shigella spp., Entamoeba histolytica
and others.
Saccharomyces boulardii and treatment of Helicobacter pylori-associated diseases
Eradication of Helicobacter pylori
involves taking two (most often) antibiotics, which also affect the entire microflora of the patient and quite often the side effect is various imbalances in microbiocenoses, which can manifest themselves in the form of antibiotic-associated diarrhea and other conditions.
The conciliation conference "Maastricht-IV" in the final document, however, gave this Regulation low levels of evidence and validity. Comments to this Statement noted that encouraging results were obtained from meta-analyses of studies using Saccharomyces boulardii
, and that these drugs are likely to reduce the incidence of side effects, especially diarrhea, and indirectly may help increase the eradication rate (Isakov V.A.).
Position of the World Gastroenterological Organization
The World Gastroenterological Organization in its material Probiotics and prebiotics. Practical recommendations. 2008 notes the effectiveness of the use of drugs containing strains of Saccharomyces boulardii
in the treatment of the following diseases:
- Acute diarrhea in children
.
Saccharomyces boulardii
has been shown to be effective in reducing the severity and duration of acute infectious diarrhea in children. Their oral use reduces the duration of diarrhea in children by approximately 1 day. The evidence obtained in studies of viral gastroenteritis is more convincing compared to bacterial or parasitic infection. An important issue is the timing of drug use. - Antibiotic-associated diarrhea
.
There is evidence of the effectiveness of treatment using Saccharomyces boulardii.
Medicines containing the active substance Saccharomyces boulardii
According to the pharmacological index, they belong to the groups “Drugs that normalize intestinal microflora”, “Antidiarrheal drugs” and “Other antimicrobial, antiparasitic and anthelmintic drugs”.
According to ATC - to the group “Antidiarrheal microorganisms”, where they are designated under the code A07FA02 as “Saccharomycetes Boulardii”. Antidiarrheal medicine with the active substance Saccharomyces boulardii, registered in Russia - Enterol. In the USA, a similar drug is sold under the trade name Florastor.
The FDA has not assigned a fetal risk category to Saccharomyces Boulardii.
In European countries and the United States, dietary supplements containing probiotic strains of Saccharomyces boulardii
and positioned as antidiarrheals: DiarSafe, Ultra Levure, OptiBac.
On the website in the literature catalog there is a section “Probiotics, prebiotics, synbiotics, symbiotics”, containing articles on the treatment of diseases of the gastrointestinal tract with probiotics, prebiotics and synbiotics.
Saccharomycetes Boulardii in the taxonomy of biological species
BULARDI sucromes are the genus of sugarmmacetes (Latin Saccharmyces
), which is part of the Sakhakromycetaceae family
,
Sakhakomitales
,
Sakhakomitseta
class
Coharomycetina
), ASCOMICTA Department ( Ascomycota
) , Kingdom of Mushrooms (
Fungi
) .
Saccharomycetes Boulardii has contraindications, side effects and application features; consultation with a specialist is necessary.
Back to section