Indications for use
- Surgery: induction of anesthesia, premedication, potentiation of general and regional anesthesia; neuroleptanalgesia (simultaneously with fentanyl); providing a sedative effect, eliminating vomiting and pain in the postoperative period, vomiting and nausea during surgical and diagnostic procedures;
- Therapy: shock and pain in injuries, severe attacks of angina, myocardial infarction, hypertensive crisis, pulmonary edema;
- Psychiatric practice: hallucinations, psychomotor agitation.
Contraindications
- Coma;
- Extrapyramidal disorders;
- Increased QT interval on ECG;
- Severe depression;
- Hypokalemia;
- C-section;
- Arterial hypotension;
- Age up to 2 years;
- Hypersensitivity to the components of the drug and morphine derivatives.
The use of Droperidol by pregnant women is possible only in cases where the expected benefit to the health of the mother is higher than the potential risk to the fetus. If it is necessary for women to use the drug during lactation, breastfeeding should be stopped.
Prolonged QT interval, TdP and LQTS
QT prolongation is a significant patient safety concern. The QT interval refers to the time from ventricular depolarization to the end of ventricular repolarization [8]. The QT interval is often corrected to the frequency of the corrected QT interval (QTc interval) through a mathematical formula known as the Bazett formula (QTc = QT/v R interval - R interval). Calculation of the QTc interval takes into account variables such as age, heart rate and gender [9]. According to Moss, “modern research provides an improved definition of QTc prolongation and estimates that the risk of malignant ventricular arrhythmias is exponentially related to the length of the QTc interval” [8]. However, Viskin believes that the actual QT interval is the best prognostic criterion for arrhythmogenic lesions before the QTc interval when significant bradycardia occurs [10]. A prolonged QT interval of more than 0.44 sec is considered pathological and is one of the main components that leads to the development of TdP [9]. A prolonged QTc interval on a standard ECG is often associated with malignant ventricular dysrhythmia, leading to syncope or death. QTc prolongation is only one of many risk factors for TdP, and the prolongation is often not impressively long. A patient with a prolonged interval in combination with metabolic disorders, left ventricular dysfunction and atrial fibrillation has an increased risk of developing malignant ventricular dysrhythmia [11]. The exact mechanism by which TdP develops is unclear. However, there is evidence that the onset of TdP may be related to several mechanisms.
TdP is a life-threatening ventricular tachycardia that is often associated with a prolonged QTc interval, hypokalemia, hypomagnesemia, and drugs known to prolong the QTc interval [12]. Other risk factors include bradycardia, female gender, congestive heart failure, congenital LQTS, and changes in T-wave morphology [10]. TdP occurs if ? 6 consecutive beats of polymorphic ventricular dysrhythmias occur [13], and the frequency ranges from 150 to 250 beats per minute [14]. TdP is often detected by the pause that precedes the dysrhythmia and by the wave of the QRS complex around the axis [10]. Another characteristic feature that often accompanies TdP is a change in the morphology of the T wave and the development of the U wave during strokes only to the point of dysrhythmia [13].
LQTS is a congenital syndrome associated with TdP that is characterized by a long QT interval in patients with a structurally normal heart [15]. LQTS can be classified as acquired and inherited forms, both of which are associated with TdP. TdP often resolves spontaneously, causing mild presyncope symptoms. However, TdP can sometimes worsen to ventricular fibrillation and is therefore potentially fatal. TdP is not always preceded by a prolonged QT interval. In fact, sources such as bradycardia, hypokalemia and hypoxia should be excluded [14]. A longitudinal study including 3343 individuals where one or more family members were found to have LQTS found a significant association with death if QTc, history of heart attack, or heart rate were measured [15].
In LQTS, disruption of the ion channels of the myocyte cell membrane leads to an excess of positive ions, prolonging the QT interval. Risk factors for death in LQTS include symptoms in infancy, deafness, history of cardiac arrest, and lack or absence of β-blocker therapy [10]. β-blocker therapy has traditionally been used as first-line therapy for LQTS.
Treatment of TdP is critical. TdP is known to progress to ventricular fibrillation [14]. Decreased oxygen delivery to the myocardium follows the development of TdP. Ventricular fibrillation can occur as a result of decreased oxygen delivery. Reduced blood flow causes acute ischemic disorders in the myocardium, leading to mechanical, electrical and chemical disintegration of heart cells. Cardiac arrest can occur quickly. If TdP remains untreated, long-term organ damage and death can occur [16].
Treatment of TdP includes defibrillation, intravenous magnesium, and possibly lidocaine infusion [10]. Treatment of metabolic disorders such as hypokalemia and hypomagnesemia is of primary importance. Discontinuation of drugs that have prodysrhythmic properties should be carried out immediately [17]. Ventricular dysrhythmias are associated with sudden death in more than 80% of cases. Early identification and treatment of dysrhythmia is critical for patient survival [16].
Directions for use and dosage
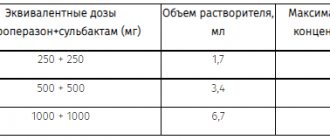
The dose of Droperidol is determined individually, taking into account the nature of the disease, age, general physical condition, body weight, drugs used simultaneously, and the type of anesthesia to be performed.
For premedication, adults are administered 2.5-5 mg of Droperidol intramuscularly 15-45 minutes before the start of surgery; children - at the rate of 100 mcg/kg.
For adults, the drug is prescribed in a dose of 15-20 mg (intravenously) for induction of anesthesia. For children, intravenous (at a dose of 200-400 mcg/kg) or intramuscular (at a dose of 300-600 mcg/kg) administration is possible.
To maintain anesthesia during long-term operations, repeated intravenous administration of Droperidol at a dose of 2.5-5 mg is possible.
In the postoperative period, adults are prescribed 2.5-5 mg intramuscularly every 6 hours.
Links
- United States Food and Drug Administration. Inapsine dear doc letter, December 4, 2001. Available at: https:// www.fda.gov/medwatch/safety/2001/inapsine.htm. Accessed March 07, 2002.
- Young D. FDA advisory panel discusses droperidol concerns. Am J Health Syst Pharm. 2004; 61: 219-222.
- American Regent Laboratories, Inc. Droperidol Injection, USP product insert. Reference number IN9702. Revised 1/02.
- Drolet B., Zhang S., Deschenes D., et al. Droperidol lengthens cardiac repolarization due to block of the rapid component of the delayed rectifier potassium current. J Cardiovascular Electrophysiol. 1999; 10: 1597-1604.
- Kao LW, Kirk MA, Evers SJ, et al. Droperidol, QT prolongation, and sudden death: What is the evidence? Ann Emerg teed. 2003; 41: 546-558.
- Pandit SK, Kothary SP, Pandit UA, et al. Dose-response study of droperidol and metoclopramide as antiemetics for outpatient anesthesia. Anesth Analg. 1999; 68: 789-802.
- Caldwell MA Pharmacology of intravenous agents. In: LW, ed. McIntosh Essentials of Nurse Anesthesia. St Louis: McGraw-Hill; 1997: 61.
- Moss A. QTc interval prolongation: is it beneficial or harmful? Am J Cardiol. 1993; 72:23B-25B.
- Lischke V., Bhene M., Doelken P., et al. Droperidol causes a dose-dependent prolongation of the QT interval. Anesth Analg. 1994; 79: 983-986.
- Viskin S. Long QT syndromes and torsades de pointes. Lancet. 1999; 354: 1625-1633.
- Morganroth J. QTc interval prolongation: Is it beneficial or harmful? Am J Cardiol. 1993; 72: 1B-3 V.
- Faigel DO, Metz DC, Kochman ML Torsades de pointes complicating the treatment of bleeding esophageal varices: Association with neuroleptics, vasopressin, and electrolyte imbalance. Am J Gastroenterol. 1995; 90:822-824.
- Gbadebo TD, Trimble RW, Khoo MSC, et al. Calmodulin inhibitor ?-7 unmasks a novel electrocardiographic parameter that predicts initiation of torsades de pointes. Circulation. 2002; 105: 770-774.
- Tan HL, Hou CJY, Lauer MR, et al. Electrophysiologic mechanisms of the long QT interval syndromes and torsades de pointes. Ann Intern Med. 1995; 122: 701-714.
- Moss A, Schwartz PJ, Crampton RS, et al. The long QT syndrome prospective longitudinal study of 328 families. Circulation. 1991; 84: 1136-1144.
- Holmberg S., Chamberlain DA Cardiac arrest and cardiopulmonary resuscitation. In: Julian DG, Camm AJ, Fox KA, eds. Diseases of the Heart. Philadelphia: Saunders; 1996: 1459-1481.
- White RD Cardiopulmonary resuscitation: Basic and advanced cardiac life support. In: Miller RD, ed. Anesthesia. Philadelphia: Churchill-Livingstone; 2000: 2533-2559.
- Buckley NA, Sanders P. Cardiovascular adverse effects of antipsychotic drugs. Drug safety. 2000; 23: 215-228.
- Lawrence KR, Nasraway SA Conduction disturbances associated with administration of butyrophenone antipsychotics in the critically ill: A review of the literature. Pharmacotherapy. 1997; 17: 531-537.
- Reilly JG, Ayis SA, Ferrier IN, et al. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet. 2000; 355: 1048-1052.
- White P.F., Song D., Abrao J., et al. Effect of low-dose droperidol on the QT interval during and after general anesthesia. Anesthesiology. 2005; 102: 1101-1105.
- Charbit B., Albaladejo P., Funck-Brentano C., et al. Prolongation of QTc interval after postoperative nausea and vomiting treatment by droperidol or ondansetron. Anesthesiology. 2005; 102: 1094-1100.
- Horowitz BZ, Bizwi K., Moreno R. Droperidol-behind the black box warning. Acad Emerg Med. 2002; 9: 615-618.
- University of Arizona Center for Education and Research on Therapeutics. Drugs to be avoided by congenital long QT patients. Available at: https://www.torsades.org/. Accessed March 15, 2005.
Side effects
When using Droperidol, it is possible to develop disorders of certain body systems:
- Central nervous system: drowsiness, dysphoria in the postoperative period and, conversely, when using high doses - fear, motor excitability, anxiety; rarely – extrapyramidal symptoms; in some cases in the postoperative period – depression, hallucinations;
- Cardiovascular system: tachycardia and moderate arterial hypotension (usually no special therapy is required); in very rare cases - arterial hypertension (most likely when combined with fentanyl or other parenterally administered analgesics);
- Digestive system: dyspepsia, loss of appetite, nausea; rarely – transient liver dysfunction, jaundice;
- Allergic reactions: rarely - dizziness, anaphylactic reactions, trembling, bronchospasm, laryngospasm.
Dear Konstantin Mikhailovich!
Many of our colleagues throughout Russia and you, of course, know about the completely intolerable, absurd situation that arose many years ago with the legal possibility (or rather, impossibility) of using fentanyl when using epidural analgesia/anesthesia.
As is known, fentanyl was synthesized in Belgium in 1959 by Paul Janssen for use as a component of a new method of anesthesia called neuroleptanalgesia - NLA (De Castro and Mundeleer, 1959). According to the idea of NLA, for its clinical implementation it was necessary to have a powerful and short-acting analgesic. This is how fentanyl was born. In the USSR, fentanyl was synthesized in the 60s of the last century by Edwards Lavrinovichs in the laboratory of the Academy of Sciences of the Latvian SSR, and the drug was packaged in Lithuanian. Fentanyl was introduced into domestic medical practice by order of the USSR Ministry of Health dated December 13, 1972. After the collapse of the USSR, the Moscow Endocrine Plant Federal State Unitary Enterprise began supplying Russian medical institutions with fentanyl, which it does to this day (the last renewal of the registration certificate was 12/12/19). In addition to him, the Federal State Unitary Enterprise “State Plant of Medical Preparations” also has the right to produce fentanyl (the last re-issuance of the registration certificate was on November 23, 2017).
As you and all Russian colleagues are well aware, the instructions for the drug fentanyl, created on the basis of these registration certificates, state that fentanyl is intended for intravenous and intramuscular administration. Epidural administration is not provided. The motives and authors of such a restriction are unknown.
But morphine has been approved for administration into the epidural and subarachnoid space since Soviet times. By the way, I was surprised to find that in the instructions for promedol (trimeperidine), the relatively recently existing permission to administer it into the epidural space mysteriously disappeared. Well, God bless him, with promedol, but the absence in the instructions for fentanyl of an indication of the possibility of epidural administration is direct and significant harm and unjustified torment of the mass of our fellow citizens who are faced with the misfortune of undergoing traumatic surgical interventions, especially in the chest and abdominal cavity.
Having a good knowledge of the literature on the topic raised, I cannot name a country, except Russia, where fentanyl would not be approved for routine epidural use, including Belarus, Ukraine and Kazakhstan. ESRA (European Society of Regional Anesthesia) and ASRA (American Society of Regional Anesthesia), NYSORA (New York Society of Regional Anesthesia), known throughout the world for its famous training programs, along with world-class anesthesiology guidelines (take Miller and Morgan for example), “to us,” of course, is not a decree. And it doesn’t matter that a wonderful, and sometimes the only effective method of anesthetic protection becomes much more powerful and safe with a combination of local anesthetic and fentanyl, jointly injected into the epidural space. It is well known that local anesthetic alone often does not provide a sufficient analgesic effect, and increasing its concentration only leads to difficult-to-control arterial hypotension. This is especially significant and sensitive during operations involving the risk of large blood loss, with one-lung ventilation, and, of course, in the postoperative period.
I undertake to speak about this confidently, having not only “bookish” knowledge, but also my own experience of more than 50,000 epidural analgesia in the “three-component” version, in which we combined small doses of fentanyl and adrenaline with a low-concentrated local anesthetic, usually 0.2 % ropivacaine. This is a highly effective version, slightly modified by us, proposed by Norwegian anesthesiologists H. Breivik and G. Niemi in 1998, and which has gained great popularity mainly in Northern Europe. This improved technique was preceded, beginning in late 1993, by >10,000 experiences with epidural analgesia using a combination of local anesthetic and fentanyl (without epinephrine). Initially, we did this under the powerful cover of our former director and long-time president of the Academy of Medical Sciences, Acad. M.I. Davydova. Then, taking advantage of the suddenly appeared opportunity to register the technique with Roszdravnadzor in the form of a “new medical technology” under the number FS No. 2010/339 dated September 15, 2010, we received legal grounds to inject fentanyl into the epidural space, especially since our director soon issued an order by the Oncology Research Center, prescribing the use of the technique in the presence of the indications listed in the order. Relatively recently it became known that the Ministry of Health de facto practically disavowed Roszdravnadzor’s permits for the use of “new medical technologies”, abolishing this form itself, however, with the caveat that the issued permits seem to continue to be valid and, subject to certain formalities, colleagues from other countries can use them medical institutions (free!) [N.G. Kurakova, T.N. Erivantseva. Registration or patenting of new medical technologies: selection methodology. Medical technologies, assessment and choice. 2011. No. 3 p. 49-53].
In fact, this cannot be considered a normal solution to a long-standing, important clinical (and legal) problem. Obviously, there can be only one radical and legal solution - to include in the registration documents and, accordingly, in the instructions for use of the drug “fentanyl” another indication and route of administration “into the epidural space” or “for epidural analgesia.” Only drug manufacturers can change registration documents, but who else can convince them to do this if not the All-Russian public organization “Federation of Anesthesiologists and Resuscitators”?
From my point of view, this task is clinical, legal, and moral and ethical in relation to our patients and colleagues. After all, it’s no secret that today the vast majority of anesthesiologists and resuscitators have mastered epidural analgesia/anesthesia. At the same time, some of them inject only a local anesthetic, which is often not enough, or they use a “trick” - they inject fentanyl into the epidural space, and in the protocol they write “intravenously”. Why on earth would they risk themselves for the benefit of their patients? Let's protect our patients and colleagues!
With deep respect and hope for success, with a complete lack of personal interest,
E.S. Gorobets
Doctor of Medical Sciences, Professor, Chief Scientific Consultant of the Department of Anesthesiology and Reanimatology of the Federal State Budgetary Institution "National Medical Research Center of Oncology named after. N.N. Blokhin" of the Ministry of Health of the Russian Federation
Dear Evgeniy Solomonovich, dear friends and colleagues!
The topic that Professor Gorobets raised in his letter really requires attention from our Federation. We know well how many such cases there are: everyone knows that a particular drug is widely used, produced and sold all over the world, is daily needed by anesthesiologists and intensivists in their routine practice - but in our country, for one reason or another, it has no effect. registration, or it is registered, but for the wrong indication, or for administration in the wrong way, etc. I don’t even want to list it again - starting with dantrolene (which finally seems to be registered! - I wouldn’t jinx it...) and antidigoxin and ending with konakion and phosphodiesterase type 3 inhibitors. At the same time, of course, much more expensive and therefore commercially more interesting drugs of much less importance are registered very quickly - you don’t have to look far for examples either. What to do if, according to the law, only the future beneficiary can become the initiator of registration and any subsequent changes to it?
Yes, we need the opportunity to legally administer fentanyl into the epidural space (why I say this is also clear to everyone). The question is for the Federation to develop a mechanism that encourages the management of companies - suppliers and manufacturers, primarily domestic ones - to initiate such registrations and changes that doctors urgently need. How to do this not on a one-time basis, but on a methodological basis? - I do not know yet. But among the most important strategic tasks of the FAR for the coming years, this topic is the creation of a systemic mechanism for professional influence on equipping the workplace of an ordinary anesthesiologist-resuscitator! - must take its place. In the meantime, let's ask the Pain Treatment Committee - Professor Alexey Mikhailovich Ovechkin, together with Evgeniy Solomonovich, to prepare a rationale for such a request from the FAR on behalf of our professional community.
With gratitude for the far-reaching initiative,
yours K. Lebedinsky
Interestingly, this order refers to the drug as “fentanyl (thalamonal).” In fact, “thalamonal” is a finished dosage form containing 50 mcg of fentanyl and 2.5 mg of droperidol per 1 ml, and was produced in Belgium in 10 ml bottles for NLA. It is unlikely that the authors of the order did not know that fentanyl and thalamonal are not the same thing. Apparently, they were focused on regulating the use of fentanyl as a narcotic analgesic, subject to strict registration. This inaccuracy indirectly indicates that at the time of the creation and introduction of fentanyl into clinical practice, most likely, no one even thought of the possibility of using fentanyl for other medical purposes, for example, for administration into the epidural space. But that was half a century ago!
special instructions
Droperidol should only be used in hospital settings.
The drug should be prescribed with caution to patients with functional disorders of the kidneys and liver, depression, epilepsy, as well as in conditions preceding an epileptic seizure.
In case of pheochromocytoma, tachycardia and severe arterial hypertension may develop after administration of Droperidol.
During therapy, the possibility of developing severe arterial hypotension must be anticipated. The drug can also cause a decrease in pressure in the pulmonary artery, which must be taken into account during diagnostic and surgical procedures. Patients receiving Droperidol need careful medical supervision.
The initial dose of the drug should be reduced in elderly, exhausted and physically weakened patients. When increasing the dose, you should be guided by the effect obtained.
Droperidol should be prescribed in a lower dose when used concomitantly with drugs that have a depressant effect on the central nervous system. Accordingly, after Droperidol, the doses of such drugs are also reduced.
The use of Droperidol in high doses (25 mg or more) can lead to sudden death in patients with cardiac arrhythmias due to alcohol withdrawal, hypoxia, or electrolyte imbalance.
In surgical practice, when using the drug, it is necessary to carefully monitor the parameters of the physiological state of the body. When performing epidural or spinal anesthesia, it is possible to develop a blockade of the sympathetic nervous system and intercostal nerves, which, in turn, can lead to difficulty breathing, the development of arterial hypotension and dilation of peripheral vessels.
To avoid the occurrence of orthostatic hypotension, it is recommended to be careful when transporting the patient and to avoid sudden changes in body position.
While Droperidol is in effect and for 24 hours after its use, it is necessary to avoid performing potentially hazardous work that requires rapid psychomotor reactions and high concentration.
Drug interactions
When using Droperidol simultaneously with certain medications, undesirable effects may occur:
- Drugs that have a depressant effect on the central nervous system (benzodiazepine derivatives, anesthetics, opioid analgesics, hypnotics): increased depressant effect on the central nervous system;
- Antihypertensive drugs: potentiation of their action;
- Epinephrine and other adrenergic and sympathomimetic drugs: manifestation of antagonism in their relation;
- Dopamine agonists, including bromocriptine, lisuride and levodopa: inhibition of their action.