The lack of an ideal drug for the treatment of patients with type 2 diabetes mellitus, which could provide not only adequate control of glycemic levels with a minimum number of side effects (weight gain, increased risk of hypoglycemia, negative effects on the heart, kidneys, liver), but also preserve secretory ability β-cells, forced scientists to continue searching for new opportunities to influence the cause of the onset and progression of this disease.
In search of an optimal method of treating T2DM, scientific research was aimed at studying a fundamentally new mechanism for regulating glucose homeostasis through gastrointestinal hormones called incretins .
It turned out that during an oral glucose load, insulin is secreted in much greater quantities than in response to intravenous administration of a similar amount of glucose. The difference in insulin secretion in response to oral and intravenous glucose loads is called the “incretin effect” (Figure 1).[1]
History of the discovery of incretins
The first information about incretins appeared more than 100 years ago. In 1902, Bayliss and Starling discovered that intestinal mucus contains a hormone that stimulates exocrine pancreatic secretion and named it "secretin." Four years later, in 1906, Moore published an article entitled "Treatment of Diabetes Mellitus by an Extract of the Duodenal Mucosa." [2]
In his article, the scientist suggested that “secretin” can affect not only the exocrine, but also the endocrine part of the pancreas. To confirm his hypothesis, the researcher isolated an extract of the duodenal mucosa and began to use it in the practice of treating patients with glucosuria. Below is one of the cases from his practice:
“A 25-year-old patient, a coachman, was admitted to the Liverpool Royal Infirmary on September 14, 1904, complaining of frequent urination, weight loss, excessive weakness and extreme thirst.
On examination, the patient's urine output was 110-120 oz [3-3.5 L] and contained 195 g of sugar. On September 24, the patient was prescribed 1 drachm [3.6 g] of sodium bicarbonate per day, and this therapy was maintained until November 18. On October 11, codeine therapy was started at a dose of S granules [0.03 g] 3 times a day... During this treatment, no decrease in sugar was observed, and on November 24 the patient was switched to 0.3 g of phenazone [antipyrine] three times a day .
After the administration of phenazone, the sugar level began to slowly decrease. .. until mid-January 1905 and remained stable in the range from 40 to 65 g per day. Without discontinuing phenazone, we gave the patient an extract of the duodenal mucosa... half an ounce... three times a day, and on February 8, 1905, the dose was doubled.
The amount of sugar [in the urine] remained constant for the first three weeks, but on February 28, 1905, it suddenly dropped to 32 grams. On March 2, it was already 25 grams... The amount of sugar continued to decrease, and finally, in May, sugar in the urine ceased to be detected, and polyuria disappeared. The patient returned to his work and soon stopped coming for the extract.”
However, despite the incredible success of Dr. Moore, the ending of this story turned out to be quite dramatic.
“The patient felt well until August 14, 1905... when he caught a cold at work. ..Since then he has become increasingly weaker and lost weight. On the thirteenth of October the patient returned to the infirmary, where he detected 4 to 5 percent sugar in 80 to 100 ounces of 24-hour urine. Despite the administration of the extract, no reduction in sugar levels was observed, and it was soon discovered that the patient was suffering from rapidly progressing tuberculosis, from which he died on December 17, 1905.”
Despite the fact that this case was fatal, one cannot fail to note the significant successes of Dr. Moore in therapy with the drug, which became the precursor to incretins. The very name “incretin” was proposed by La Barre in 1932 for a hormone isolated from the mucus of the upper intestine and capable of causing hypoglycemia.
The first hormone with incretin activity was isolated from an extract of pig duodenal mucus. Due to its property of inhibiting the secretion of gastric hydrochloric acid, the peptide was named “gastric inhibitory polypeptide” (GIP). It was later discovered that the main biological effect of this newly discovered peptide is glucose-dependent stimulation of insulin secretion , and therefore it was proposed to rename GIP to glucose-dependent insulinotropic polypeptide (GIP) . The site of its synthesis is the K-cells of the intestinal mucosa, mainly the duodenum and jejunum.
In 1983, Bell et al. isolated the sequence encoding two glucagon-like peptides, GLP-1 and GLP-2, from the hamster proglucagon gene. In mouse models, it was shown that it was GLP-1 that stimulated glucose-dependent insulin secretion, i.e. had incretin activity. The site of synthesis of this hormone is the endocrine L-cells of the ileal mucosa (Fig. 2).
Glucagon-like peptides, like glucagon, are products of the proglucagon gene. In the pancreas, as a result of reading this gene, glucagon is synthesized, and in the L-cells of the small intestine - GLP-1, GLP-2 and glycentin (enteroglucagon). GLP-2, despite its structural similarity to GLP-1, does not have the same biological effect: the action of GLP-2 is limited to the regulation of growth processes in the intestinal tract.
APUD-system[ | ]
Main article: Diffuse endocrine system
The term and concept of the APUD system (“APUD” is an acronym formed from the first letters of the English words a
mine - amines,
p
recursor - precursor,
u
ptake - assimilation, absorption;
d
ecarboxylation - decarboxylation) was proposed by E. Pearce (English AGE Pearse) in 1969, based on the ability of cells of the APUD system to assimilate amine precursors (monoamines L-dihydroxyphenylalanine and 5-HTP), decaroboxylate them and synthesize the amines necessary for the formation regulatory peptides.[2]
Recently, instead of the term APUD system, the previously accepted synonym diffuse endocrine system
, at the same time, derivative terms such as
apudocytes
- cells that are part of the APUD system,
apudoms
- tumors resulting from apudocyte hyperplasia, are actively used in modern medical vocabulary.
Similarities and differences between GLP-1 and GIP
The release of GLP-1 and GIP depends on nutritional, neurogenic and hormonal stimuli and occurs immediately after food intake: a significant increase in incretin concentrations is observed after 10-15 minutes.
Research results have shown that the secretion of GLP-1 and GIP is stimulated by the absorption of fats and carbohydrates in the intestine. GLP-1 secretion is also influenced by protein absorption. Moreover, simple contact of these nutrients with the intestinal mucosa is sufficient to release incretin hormones from K and L cells, which leads to a rapid rise in insulin levels in the blood. However, the rise in plasma GLP-1 concentrations occurs too quickly to be due to direct activation of L cells: most of these cells are located in the distal small intestine, and it would take longer for nutrients to reach this level.
An alternative theory to explain the rate of incretin release in intestinal cells is the neurogenic regulation theory, which suggests that activation of cholinergic muscarinic receptors located on the surface of L cells causes the secretion of GLP-1. However, there is very little data confirming the presence of a vagal effect on the function of L-cells, and activation of the sympathetic nervous system completely leads to inhibition of the secretory function of L-cells.
The third hypothesis designed to explain the early onset of incretin secretion is the theory of paracrine influence. Somatostatin, released from intestinal D cells, suppresses the secretion of incretins, and inhibition of the action of somatostatin causes a sharp increase in GLP-1 levels. In animal experiments, high doses of GIP also stimulate the production of GLP-1 by L cells, although this effect has not been proven in humans.
General properties of GIP and GLP-1:
- quickly (GLP-1 - within 2 minutes, GIP within - 6 minutes) are cleaved by the enzyme dipeptidyl peptidase type 4 (DPP-4) (Fig. 3).
- GLP-1 is destroyed before it leaves the intestine, since DPP-4 is present on the surface of the endothelial cells of the capillaries of the intestinal mucosa.
- Helps increase β-cell mass (in animal models)
Differences between GIP and GLP-1:
- GIP: Does not affect the evacuation of food from the stomach
- Does not affect saturation and body weight
- Does not affect glucagon secretion by pancreatic α-cells
- Does not affect the cardiovascular system
- GIP secretion is preserved in diabetic patients
- Does not stimulate (or weakly stimulates) insulin secretion in patients with T2DM
- Slows down the evacuation of food from the stomach
Content
- 1 APUD system
- 2 Apudocytes of the gastroenteropancreatic endocrine system 2.1 Endocrine cells of the stomach
- 2.2 Endocrine cells of the duodenum and jejunum
- 2.3 Endocrine cells of the ileum and colon
- 2.4 Endocrine cells of the pancreas
- 3.1 Incretins
- 4.1 Vipoma
Targets and mechanism of action of GLP-1
As follows from the data presented, GLP-1 is more attractive than GIP for the treatment of patients with T2DM. In this regard, the vast majority of research is currently aimed at studying the biological activity and creating analogues of GLP-1 as a therapy for T2DM.
A detailed study of the effects of GLP-1 showed its direct effect not only on the pancreas, but also on the tissues of the liver, stomach, brain, and heart muscle (Fig. 4).
The effects of GLP-1 are mediated by receptors (rGLP-1) that have been found in the pancreatic islets, kidneys, heart, stomach, lungs, and in the peripheral and central nervous systems.
Effect of GLP-1:
- On pancreatic β-cells
GLP-1 has multiple effects on the endocrine pancreas, but its principal effect is to potentiate insulin secretion.
The mechanism of this effect is as follows: contact of GLP-1 with the receptor – increase in the amount of intracellular cAMP – stimulation of protein kinase A – exocytosis of insulin granules from β-cells.
It is important that stimulation of insulin secretion by glucagon-like peptide-1 is glucose-dependent in nature, i.e. GLP-1 stimulates insulin secretion only at high glycemic levels. Once plasma glucose levels fall to normal levels (approximately 4.5 mmol/L), the stimulatory effect of GLP-1 disappears.
In addition to stimulating insulin secretion, GLP-1 also affects all stages of the insulin biosynthesis process, i.e. prepares insulin reserves for its secretion, which prevents depletion of insulin reserves due to stimulation of its secretion.
Experimental studies have shown that GLP-1 affects the mass of β-cells, stimulating their proliferation and neogenesis and blocking apoptosis. However, these data currently have no clinical confirmation in humans.
- On pancreatic a-cells
GLP-1 causes a decrease in glucagon secretion.
This effect may be due to:
- Indirect stimulation of α-cells - through stimulation of the secretion of insulin and somatostatin.
- Direct stimulation of α-cells, since they also contain receptors for GLP-1. Confirmation of the direct effect of GLP-1 on α-cells is the fact that in patients with type 1 diabetes (in the complete absence of insulin secretion), the administration of GLP-1 also suppressed glucagon secretion and reduced glycemic levels.
Suppression of glucagon secretion under the influence of GLP-1 is also glucose-dependent.
- On cells of the gastrointestinal tract (GIT)
Stimulation of rGLP-1 in the ileum reduces gastrointestinal motility, slows gastric emptying and glucose absorption. As a result, a decrease in the level of postprandial glycemia is observed. This effect is called the “intestinal brake.”
The essence of the phenomenon is that food that enters the distal parts of the intestine can inhibit the motility and secretory activity of the upper intestines and stomach. The mechanism of this phenomenon is associated with the activation of afferent fibers of the vagus nerve and inhibition of the passage of impulses along the efferent ones. A study in healthy volunteers showed that intravenous administration of GLP-1 caused a dose-dependent reduction in the rate of gastric emptying. As a result, postprandial blood glucose levels decrease down to basal levels.
It is assumed that the decrease in postprandial glucose concentration with the introduction of GLP-1 is achieved mainly due to inhibition of gastric emptying, and not only due to an increase in insulin synthesis by the pancreas.
- To the heart muscle
Receptors for GLP-1 have been discovered in the myocardium, which has found its application in clinical practice. Studies in mice lacking rGLP-1 have shown reduced left ventricular contractility and diastolic dysfunction, and trials in dogs have shown that GLP-1 administration can improve cardiac function in animals with heart failure by increasing cardiac output.
In animals with myocardial ischemia, the ability of GLP-1 to reduce infarct size has been demonstrated, suggesting a possible cardioprotective role for this incretin.
In addition, a beneficial effect of GLP-1 on coronary blood flow, not mediated by rGLP-1, was found. Thus, in an experiment on dogs with dilated cardiomyopathy, it was shown that the GLP-1 metabolite (GLP-1 (9-36)), formed as a result of the destruction of GLP-1 by the enzyme DPP-4, increases the flow of glucose into the myocardium, which improves left ventricular function in these animals. The same metabolite has the ability to cause NO-dependent vasodilation of coronary vessels and, as a result, improve blood supply to the heart muscle.
- On brain tissue
Since rGLP-1 was found in the nuclei of the hypothalamus, which are responsible for the process of satiety, the effect on these receptors can influence eating behavior. In rats, administration of GLP-1 into the ventricles of the brain helped to reduce the time of food intake and its quantity, while the opposite effect was observed with the administration of GLP-1 antagonists.
Subsequent trials showed that central administration of GLP-1 agonists caused a decrease in the frequency of meals and water intake, leading to weight loss. Similar results were obtained in clinical trials of GLP-1 agonists in healthy people, diabetics, and obese individuals, where peripheral subcutaneous administration of these drugs led to rapid onset of satiety, a decrease in the amount of food consumed, and weight loss.
- Liver, skeletal muscle, adipose tissue
In the liver, GLP-1 inhibits gluconeogenesis, and in adipose and muscle tissue it promotes glucose uptake. However, these effects have less effect on reducing glycemia compared to the regulation of insulin and glucagon secretion.
- On bone tissue
The experiment showed that GLP-1 controls the process of bone tissue resorption. In the absence of rGLP-1, mice exhibited cortical osteopenia and increased numbers of osteoclasts and markers of bone resorption.
- These effects were eliminated in the presence of calcitonin, indicating that the protective effect of GLP-1 on bone tissue occurs through a calcitonin-dependent mechanism.
The role and place of incretins in achieving comprehensive glycemic control
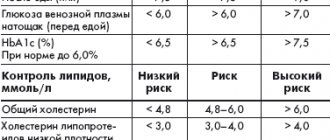
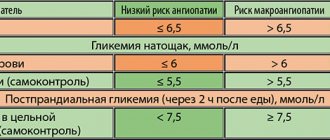
In 2010, the number of people with diabetes worldwide, according to the International Diabetes Federation (IDF), was 285 million people, and 85–90% of them suffer from type 2 diabetes. IDF forecasts regarding the rate of increase in the prevalence of diabetes in the world are disappointing - by 2030, the total number of patients with diabetes, according to rough estimates, will increase by 1.5 times and amount to 438 million people [1]. The relevance of the problem of type 2 diabetes is due not only to the increase in its prevalence, but also to the serious complications that develop against the background of this disease. It has been proven that type 2 diabetes is associated with a significant risk of developing primarily cardiovascular diseases (CVD): coronary heart disease, stroke, arterial hypertension, obliterating atherosclerosis of the arteries of the lower extremities. The risk of heart attack and stroke in patients with type 2 diabetes is 2–4 times higher, and the risk of lower limb amputation is 10–15 times higher compared to patients without diabetes. In 75% of cases, CVDs are the cause of death in patients with type 2 diabetes. A relationship has also been established between type 2 diabetes and the risk of chronic kidney disease and blindness [2]. Since the main pathophysiological disorder in diabetes leading to the development of complications is chronic hyperglycemia, treatment of diabetes aimed at controlling glycemia, as shown by large international studies, significantly reduces the risk of developing micro- and macrovascular complications in patients with both type 1 diabetes and Type 2 diabetes [3,4]. For many years, the traditional and generally accepted indicators of carbohydrate metabolism compensation, which doctors relied on when choosing treatment tactics, were fasting plasma glucose (FPG) and glycosylated hemoglobin (HbA1c). The importance of HbA1c control was confirmed in the DCCT (Diabetes Control and Complications Trial) study in patients with type 1 diabetes. The study results showed that continuous glycemic control (average HbA1c level ~7% over 6.5 years) led to a reduction in the risk of developing microalbuminuria by 39%, proteinuria by 54%, neuropathy by 60%, and retinopathy by 76% ( Fig. 1) [4]. The large UKPDS (United Kingdom Prospective Diabetes Study) study demonstrated that a 1% reduction in HbA1c resulted in a 21% reduction in the risk of developing any type 2 diabetes-related complications, including a 14% reduction in the risk of myocardial infarction, a 12% reduction in stroke, and microvascular complications – by 37% (p <0.0001) [3]. However, there are a number of factors limiting the use of HbA1c monitoring in clinical practice - some conditions in which HbA1c measurement can give false results, both overestimated and underestimated (Table 1) [5–9]. In addition, HbA1c does not reflect the variability of glycemic levels throughout the day. In patients with the same HbA1c values, fluctuations in glycemia during the day, as shown by the results of long-term glucose monitoring, can vary greatly - from hypoglycemia to hyperglycemia. Glycemic variability is primarily a predictor of severe hypoglycemia, especially in patients with diabetes on insulin therapy [10]. Typically, hypoglycemia is a serious complication of intensive treatment of type 2 diabetes. For example, a recent meta-analysis of 13 studies including more than 34,000 patients showed that intensive therapy for type 2 diabetes increases the risk of hypoglycemia by more than 2 times compared with standard therapy [11]. At the same time, data from the VADT study (the Veteran Affairs Diabetes Trial) indicate that hypoglycemia is an even more significant risk factor for the development of CVD than HbA1c values, HDL cholesterol, a history of CVD, and the patient’s age [12]. The significant contribution of hypoglycemia to the risk of developing CVD can be confirmed by the results of an experiment demonstrating that hypoglycemia is accompanied by the development of myocardial ischemia: during CGMS (Continuous Glucose Monitoring System) and 24-hour ECG monitoring against the background of confirmed hypoglycemic conditions, including asymptomatic ones, the development of episodes of angina pectoris was noted and the appearance of ECG abnormalities characteristic of ischemia [13]. In addition, hypoglycemia plays a serious role in shaping patient adherence to treatment - fear of hypoglycemia very often leads to the patient’s refusal of recommended treatment and, as a consequence, to the lack of adequate glycemic control [14]. According to the literature, glycemic variability may also be important for the development of oxidative stress and, as a result, the progression of complications of diabetes, which has been demonstrated in vitro, in animal experiments and in studies in patients with type 2 diabetes. There was also interesting evidence that glycemic variability is associated with increased mortality in patients without diabetes, but who are in serious condition due to other diseases [10]. Postprandial glycemic control (PPG) is now receiving increasing attention due to the accumulation of compelling research evidence for its role in glycemic control and the development of late complications of diabetes. Data from a number of epidemiological studies show that patients with type 2 diabetes and HbA1c <7.0% often have high levels of postprandial glycemia [15]. For example, in the study of the US National Health Registry NHANES III (The Third National Health and Nutrition Examination Survey) it was found that 40% of patients with type 2 diabetes receiving oral glucose-lowering drugs and having good glycemic control (HbA1c <7.0 %), PPG levels are determined to be more than 11.1 mmol/l with FPG values less than 6.7 mmol/l. Moreover, the same study showed that all patients with HbA1c from 7.0 to 7.9% had PPG values greater than 11.1 mmol/l [16]. What is the significance of PPG for patients with type 2 diabetes? It has been established that PPG is an independent and more significant risk factor for CVD than FPG. The DECODE (The Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe) study, which analyzed data from 25,000 patients with diabetes, found that PPG is a more powerful predictor of CVD and all-cause mortality (Fig. 2) [17]. PPG has been found to trigger a cascade of metabolic disorders, including oxidative stress, impaired vascular reactivity, hypercoagulability and endothelial dysfunction, which lead to the progression of atherosclerosis and the development of macrovascular complications of type 2 diabetes [18–20]. In addition, a close relationship was found between PPG and the risk of developing retinopathy, increased carotid artery intimal thickness, increased risk of pancreatic cancer, and impaired cognitive function in elderly patients with type 2 diabetes [21–23]. It is also worth noting that the contribution of PPG to HbA1c increases as glycemic control improves and HbA1c approaches normal values [24]. Considering all of the above, it should be noted that effective management of type 2 diabetes is impossible without comprehensive glycemic control, which involves monitoring three indicators of glycemic levels - FPG, PPG and HbA1c (the so-called glucose triad). When choosing treatment tactics for a patient with type 2 diabetes, the doctor should focus on all three indicators; it is this approach that will ensure the effectiveness and safety of the recommended treatment and allow the patient to avoid severe complications of type 2 diabetes. Currently, for the treatment of type 2 diabetes, there are several classes of oral hypoglycemic drugs with different mechanisms of action, safety and effectiveness: sulfonylurea derivatives and meglitinides, which stimulate insulin secretion; metformin, which suppresses the processes of glucose synthesis by the liver; thiazolidinediones, which increase insulin sensitivity in peripheral tissues; α-glucosidase inhibitors, which reduce the absorption of glucose in the small intestine; drugs with an incretin effect that restore physiological insulin secretion. However, despite a wide range of medications available, the majority of patients worldwide do not achieve glycemic targets. There are several barriers to achieving optimal glycemic control in patients with type 2 diabetes. One of them is the natural progressive course of type 2 diabetes, which is characterized by increasing β-cell dysfunction and decreased insulin secretion. These pathophysiological disorders require constant intensification of glucose-lowering therapy to maintain optimal glycemic control. Another barrier to achieving glycemic control is treatment complications such as hypoglycemia, weight gain with long-term use, unsatisfactory tolerability and, as a result, low patient adherence to recommended treatment. The emergence of a new class of incretin drugs has become a real breakthrough in modern diabetology, because this class of drugs throughout the world has high hopes for overcoming barriers to achieving adequate glycemic control - β-cell dysfunction and treatment complications. Drugs with an incretin effect are a new class of hypoglycemic drugs, the main mechanism of action of which is a glucose-dependent increase in insulin secretion and suppression of glucagon secretion. Thanks to the mechanism of action, drugs in this group allow you to safely control all indicators of the glucose triad - HbA1c, FPG and PPG, which, of course, is their advantage for both the doctor and the patient, since only comprehensive glycemic control, as mentioned above, can significantly reduce the risk development of severe complications of type 2 diabetes – primarily CVD. Another advantage of drugs in this group is their good safety profile: they are characterized by a low risk of hypoglycemia and no negative effect on body weight [25]. So what is this incretin effect? Incretins are biologically active substances, hormones produced in intestinal cells in response to food intake and responsible for 50–70% of postprandial insulin secretion in healthy individuals. It is this contribution of incretins to insulin secretion that is called the incretin effect (Fig. 3) [26]. The most significant role in insulin secretion and carbohydrate metabolism is played by glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP). GIP is secreted by K cells of the duodenum and jejunum in response to meals rich in carbohydrates and fats. In addition, GIP is involved in lipid metabolism in adipocytes and has a proliferative effect on β cells. GLP-1 is secreted by L cells of the ileum and colon. GLP-1 has a variety of effects on carbohydrate metabolism, including glucose-dependent stimulation of insulin secretion, glucose-dependent suppression of glucagon secretion, decreased appetite and gastric emptying, and possibly improved insulin sensitivity. In addition, GLP-1 increases the transcription of the insulin gene and is involved in all stages of insulin biosynthesis [27–29]. Animal studies have demonstrated that GLP-1 increases the mass of β-cells and suppresses their apoptosis [30]. Incretins are rapidly destroyed by the enzyme dipeptidyl peptidase-4 (DPP-4), which is found not only in the vascular endothelium of the intestinal mucosa, but is also widely represented in many tissues of the human body, including the lungs, brain, kidneys, adrenal glands, pancreas, and intestines. and lymphocytes. GLP-1 is destroyed almost immediately after secretion. The half-life for GLP-1 is less than 2 minutes, for GIP - 5-6 minutes. [27–29]. However, it has been found that in patients with type 2 diabetes, the incretin effect is reduced, which may be due to impaired incretin secretion processes, their accelerated metabolism, or the insensitivity of cellular receptors to their action. An interesting fact is that in patients with type 2 diabetes, the concentration of GLP-1 decreases primarily while maintaining the biological activity of this incretin, while the levels of GIP remain within normal values or close to normal, but it loses all effects on insulin secretion ( Fig. 4) [31,32]. It was these data that made it possible to develop and introduce into clinical practice a class of drugs whose hypoglycemic mechanism is based on the effects of GLP-1. In clinical practice, drugs are used that are GLP-1 agonists or mimetics, that is, they have the effects of GLP-1 on the secretion of insulin and glucagon, but are resistant to the action of the DPP-4 enzyme, and DPP-4 inhibitors, the use of which causes an increase in concentrations of natural GLP-1 by 1.5–3 times and, as a consequence, glucose-dependent stimulation of insulin secretion and suppression of glucagon secretion. Currently, two GLP-1 agonists resistant to the action of DPP-4 are available to doctors and patients in Russia - liraglutide, exenatide and several DPP-4 inhibitors - saxagliptin, sitagliptin, vildagliptin. The introduction into clinical practice of new classes of glucose-lowering drugs that provide comprehensive glycemic control is a promising approach to the treatment of type 2 diabetes.
References 1. International Diabetes Federation. IDF Diabetes Atlas, 4th ed. Brussels, Belgium: International Diabetes Federation, 2009. https://www.idf.org/diabetesatlas, accessed July 6th 2011. 2. US Department of Health and Human Services, National Diabetes Information Clearinghouse. National Diabetes Statistics, 2007. NIH Publication No. 08–3892. https://diabetes.niddk.nih.gov/dm/pubs/statistics/DM_Statistics.pdf. Last updated: June 2008. Accessed October 19, 2010. 3. Stratton IM, Adler AI, Neil AW et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. // BMJ. 2000; 321:405–412. 4. The DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long–term complications in insulin–dependent diabetes mellitus. // N Engl J Med. 1993; 329:977–986. 5. Koskinen LK et al. Does uremia interfere with HbA1c results in the FPLC method with Mono S cation exchanger? // Clin Chem Acta. 1998 May 8; 273(1): 69–79. 6. Wang X. et al. Hemoglobin A1c levels in non-diabetic patients with end-stage renal disease (ESRD) receiving hemodialysis. // J Endocrinol Invest. 2004 Sep; 27(8): 733–5. 7. Coban E. et al. Effect of iron deficiency anemia on the levels of hemoglobin A1c in nondiabetic patients. // Acta Haematol. 2004 112(3): 126–8. 8. Camargo JL et al. Conditions associated with very low values of glycohaemoglobin measured by an HPLC method. // J Clin Pathol 2004 Apr; 57(4): 346–9. 9. Tseng CL et al. Seasonal patterns in monthly hemoglobin A1c values. // Am J Epidemiol. 2005 Mar 15; 161(6): 565–74. 10. Siegelaar SE, Holleman F., Hoekstra JB, DeVries JH Glucose variability; does it matter? // Endocr Rev. 2010 Apr;31(2):171–82. Epub 2009 Dec 4. 11. Boussageon R., Bejan-Angoulvant T., Saadatian-Elahi M. et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta–analysis of randomized controlled trials. // BMJ. 2011 Jul 26; 343:d4169. 12. Skyler S., Bergenstal R., Bonow R. et al. Intensive Glycemic Control and the Prevention of Cardiovascular Events: Implications of the ACCORD, ADVANCE and VA. // Diabetes Trials Diabetes Care. 2009; 32(1):187–192. 13. Desousza LC et al. Accotiation of Hypoglycemia and Cardiac Ischemia. // Diabetes Care. 2003; 26:1458–8. 14. Marrett E. et al. Patient–reported outcomes in a survey of patients treated with oral antihyperglycaemic medications: associations with hypoglycaemia and weight gain. // Diabetes, Obesity and Metabolism. 2009; 11: 1138–1144. 15. Gerich JE Clinical significance, pathogenesis, and management of postprandial hyperglycemia. //Arch. Intern. Med. 163, 1306–1316 (2003). 16. Saydah S., Loria C., Eberhardt M., Brancati F. Subclinical states of glucose intolerance and risk of death in the US. // Diabetes Care. 2001; 24:447–453. 17. The DECODE Study Group. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. // Lancet. 1999; 354:617–621. 18. Ceriello A., Bortolotti N., Motz E. et al. Meal induced oxidative stress and low density lipoprotein oxidation in diabetes: the possible role of hyperglycaemia. //Metabolism. 1999; 48:1503–08. 19. Kawano H., Motoyama T., Hirashima O., et al. Hyperglycaemia rapidly suppresses flow mediated endothelium dependent vasodilation of brachial artery. // J Am Coll Cardiol. 1999;34:146–54. 20. Ceriello A. Coagulation activation in diabetes mellitus: the role of hyperglycaemia and therapeutic prospects. // Diabetologia. 1993; 36:1119–25. 21. Larsson SC, Bergkvist L., Wolk A. Consumption of sugar and sugar-sweetened foods and the risk of pancreatic cancer in a prospective study. // Am J Clin Nutr 2006; 84(5):1171–1176. 22. Abbatecola AM, Rizzo MR, Barbieri M. et al. Postprandial plasma glucose excursions and cognitive functioning in aged type 2 diabetics. // Neurology 2006; 67(2):235–240. 23. Hanefeld M., Koehler C., Schaper F. et al. Postprandial plasma glucose is an independent risk factor for increased carotid intima–media thickness in non–diabetic individuals. // Atherosclerosis. 1999;144(1):229–235. 24. Monnier L., Lapinski H., Collette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of Type 2 diabetic patients: variations with increasing levels of HbA1C. // Diabetes Care. 26, 881–885 (2003). 25. Deacon CF Dipeptidyl peptidase–4 inhibitors in the treatment of type 2 diabetes: a comparative review. // Diabetes, Obesity and Metabolism. 2011;13:7–18. 26. Nauck MA, Homberger E, Siegel EG et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C–peptide responses. // J Clin Endocrinol Metab. 1986; 63:492–498. 27. Baggio LL, Drucker DJ Biology of incretins: GLP–1 and GIP. // Gastroenterology. 2007; 132:2131–2157. 28. Holst JJ The physiology of glucagon-like peptide 1. // Physiol Rev. 2007; 87:1409–1439. 29. Holst JJ, Vilsboll T., Deacon CF The incretin system and its role in type 2 diabetes mellitus. // Mol Cell Endocrinol. 2009; 297:127–136. 30. Drucker DJ Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. // Mol Endocrinol. 2003;17:161–171. 31. Amori RE, Lau J., Pittas AG Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. // JAMA. 2007; 298(2):194–206. 32. Toft–Nielsen MB, Damholt MB, Madsbad S. et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. // J Clin Endocrinol Metab. 2001; 86(8):3717–3723.
Incretin effect in type 2 diabetes mellitus
In patients with T2DM and obesity, a significant decrease in the incretin effect is observed, i.e. decreased insulin secretion in response to an oral glucose load while its secretion remained intact in response to intravenous glucose (Fig. 5).
A decrease in the incretin effect entails a violation of the insulin response to carbohydrate intake and, accordingly, an increase in blood glucose levels.
When studying the reason for the decrease in incretin response in patients with T2DM, it was found that this is associated with lower secretion of GLP-1 (with preserved secretion of GIP). At the stage of prediabetes, a decrease in GLP-1 secretion is also observed, but less pronounced than in patients with T2DM. (Fig.6)
CONCLUSIONS:
- Incretins are gastrointestinal hormones produced in response to food intake and stimulate insulin secretion.
- The “incretin effect” is the difference in insulin secretion in response to oral and intravenous glucose loads.
- Glucose-dependent stimulation of insulin secretion and glucose-dependent suppression of glucagon secretion provided by GLP-1 are mechanisms of protection against the occurrence of hypoglycemic conditions.
- GLP-1 causes a dose-dependent decrease in the rate of gastric emptying. This results in a significant decrease in postprandial blood glucose levels. Presumably, this effect is achieved primarily by inhibiting gastric emptying, and not only by increasing insulin synthesis by the pancreas.
- Activation of rGLP, as well as the direct effect of the GLP-1 metabolite on the heart muscle, provides beneficial cardiovascular effects of GLP-1 analogues: increased cardiac output, reduced myocardial infarction area, improved coronary blood flow.
- The effect of GLP-1 on the hypothalamic nuclei contributes to the rapid onset of satiety, a decrease in the amount of food consumed and, as a result, to a decrease in body weight.
- GLP-1 reduces insulin resistance in peripheral tissues and reduces hepatic glucose production.
- GLP-1 prevents the development of osteoporosis and osteopenia.
- In patients with insulin resistance, there is a significant decrease in insulin secretion in response to an oral glucose load, while its secretion is preserved in response to intravenous glucose. A decrease in the incretin effect entails a weakening of the insulin response to carbohydrate intake and, as a result, an increase in blood glucose levels.
Sources:
- DEDOV I.I. et al. INDICATORS OF CARBOHYDRATE METABOLISM AND INCRETIN PRODUCTION IN PATIENTS WITH MORBID OBESITY, INCLUDING THOSE WHO HAVE HAD BILIOPANCREATIC BYPASS // Obesity and Metabolism. 2014. No. 1. P. 24–31.
- Moore V. On the treatment of diabetes mellitus by acid extract of duodenal mucous // Biochem J. 1906. Vol. 1. P. 28–38.
Apudoms[ | ]
Main article: Apudoms
Apudoms are tumors arising from cellular elements located in various organs and tissues (mainly islet (incretory) cells of the pancreas, cells of other parts of the gastrointestinal tract, C-cells of the thyroid gland), producing polypeptide hormones. The following types of apudom are currently described:[10]
- VIPoma;
- Gastrinoma;
- Glucagonoma;
- Carcinoid;
- Neurotensinoma;
- PPoma;
- Somatostatinoma;
- Insulinoma
Vipoma[ | ]
Main article: VIPoma
VIPoma (Werner-Morrison syndrome, pancreatic cholera, watery diarrhea-hypokalemia-achlorhydria syndrome) is characterized by the presence of watery diarrhea and hypokalemia as a result of islet cell hyperplasia or a tumor, often malignant, arising from the islet cells of the pancreas (usually the body and tail), which secrete vasoactive intestinal polypeptide (VIP). In rare cases, VIPoma may occur in ganglioneuroblastomas, which are localized in the retroperitoneal space, lungs, liver, small intestine and adrenal glands, occur in childhood and are usually benign. The size of pancreatic VIPomas is 1...6 cm. In 60% of cases of malignant neoplasms, there are metastases at the time of diagnosis.[11] The incidence of VIPoma is very low (1 case per year per 10 million people) or 2% of all endocrine tumors of the gastrointestinal tract. In half of the cases the tumor is malignant. The prognosis is often unfavorable.[12]
Gastrinoma[ | ]
Main article: Gastrinoma
With G-cell hyperplasia, gastrinoma is formed - a benign or malignant tumor localized in the pancreas, duodenum or jejunum, or even in the peripancreatic lymph nodes, in the hilum of the spleen or the wall of the stomach. This tumor produces more gastrin, hypergastrinemia occurs, which, through the mechanism of stimulation of parietal cells, causes excessive production of hydrochloric acid and pepsin. In a normal situation, G-cells under the influence of hydrochloric acid inhibit the production of gastrin, but the acidity factor does not affect G-cells with gastrin. As a result, multiple peptic ulcers of the stomach, duodenum or jejunum develop. The secretion of gastrin by gastrinomas increases especially sharply after eating.
The clinical manifestation of hypergastrinemia is Zollinger-Ellison syndrome (type 1)..[13]
Glucagonoma[ | ]
Main article: Glucagonoma
Glucagonoma is a tumor, often malignant, arising from the alpha cells of the pancreatic islets. It is characterized by migratory erosive dermatosis, angular cheilitis, stomatitis, glossitis, hyperglycemia, normochromic anemia. It grows slowly and metastasizes to the liver. Occurs 1 in 20 million between the ages of 48 and 70, most often in women.[10]
Carcinoid[ | ]
| This section is not completed. You will help the project by correcting and expanding it. |
Neurotensinoma[ | ]
| This section is not completed. You will help the project by correcting and expanding it. |
PPoma[ | ]
Main article: PPoma
PPoma is a tumor of the pancreas that secretes pancreatic polypeptide (PP). Clinical manifestations are practically absent. More often diagnosed after metastasis to the liver.[10] Treatment: surgery, chemotherapy and symptomatic. The prognosis depends on the timing of the start of treatment.
Somatostatinoma[ | ]
Main article: Somatostatinoma
Somatostatinoma is a malignant, slow-growing tumor characterized by increased levels of somatostatin. This is a rare disease, occurring in people over 45 years of age - 1 case in 40 million.[10]
There are:
- somatostatin
from pancreatic delta cells and - apudoma
, secreting somatostatin, is a tumor of the duodenum.
Diagnosis based on clinical presentation and increased levels of somatostatin in the blood. Treatment is surgical, chemotherapy and symptomatic. The prognosis depends on the timeliness of treatment.
Insulinoma[ | ]
Main article: Insulinoma