Vaccine options
Chickenpox vaccinations currently available on the market for children and adults are obtained using the so-called Oka VZV strain, which has been modified through sequential reproduction in various cell cultures. Various formulations of such live attenuated vaccines have undergone rigorous testing and have been approved for use in Japan, the Republic of Korea, the USA, and also in several European countries. Some vaccines are approved for use in children 9 months and older. In Russia, the Varilrix vaccine is used to prevent chickenpox. The Okavax vaccine is currently not used in Russia or Europe.
Indications for vaccination
For preventive purposes, children begin to be vaccinated after 1 year of age and older. It is carried out for:
- children over 2 years of age who have not had chickenpox;
- children who are over 2 years old and go to children's health camps in the summer.
In addition, vaccination is required for:
- persons who have severe chronic diseases;
- patients with acute leukemia;
- persons who receive drugs to enhance immunity;
- people who have undergone chemotherapy;
- patients who are planning a transplant operation.
Vaccination is carried out only in the absence of signs of insufficiency of cellular immunity. In addition, patients with chronic diseases are vaccinated only with a positive assessment of their health status, before transplantation; the vaccine is administered 1-2 weeks before surgery.
Principles and purposes of vaccination
Today, chickenpox (chickenpox) is one of the most contagious and common infections in the world - almost all children or young adults get it. Other than vaccination, there are no countermeasures to control the spread of chickenpox or the incidence of shingles in a susceptible community. Administering chickenpox and other vaccines at the same time to a person at different sites and using different syringes is as safe and effective as administering the same vaccines several weeks apart.
However, in order to induce the same immune response as in the case of a monovalent vaccine, the dose of the varicella component must be increased (if included in a tetravalent vaccine in combination with a measles-mumps-rubella vaccine). From the point of view of logistics and the epidemiological situation, the optimal age for chickenpox vaccination in children is 12-24 months.
Why get vaccinated?
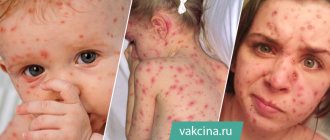
Chickenpox (also called chickenpox) is a common childhood disease. It is usually mild, but can be serious, especially in infants and adults.
- This disease causes a rash, itching, fever and fatigue.
- It can cause severe skin infections, scarring, pneumonia, brain damage, or death.
- The chickenpox virus can be transmitted from person to person through the air or through contact with fluid contained in chickenpox blisters.
- A person who has had chickenpox may, many years later, develop a painful rash called shingles.
- Before the vaccine was available, about 11,000 people were hospitalized with chickenpox each year in the United States.
- Before the vaccine was available, 100 people died from chickenpox every year in the United States.
The chickenpox vaccine can prevent chickenpox.
Most people who get the chickenpox vaccine do not get it. But if a person who has been vaccinated does get chickenpox, the disease is usually mild. People vaccinated against chickenpox have fewer blisters and fewer fevers; In addition, they recover faster.
Vaccination effectiveness
Varicella vaccinations using the Oka strain of VZV have been on the market since 1974. Positive results regarding safety, effectiveness and cost-effectiveness analyzes supported the rationale for their introduction into childhood immunization programs in a number of industrialized countries. After following study populations for 20 years in Japan and 10 years in the United States, more than 90% of immunocompetent adults vaccinated as children were still protected against varicella. In Japan and several other countries, one dose of vaccine is considered sufficient regardless of age. In the USA, 2 doses of the vaccine, administered at 4-8 week intervals, are recommended for children and adults, among whom 78% seroconverted after the first dose and 99% after the second dose of the vaccine. According to the current US vaccination schedule, children receive 2 doses of the vaccine (1st dose at 12 months, 2nd at 6 years).
In response to chickenpox vaccination, about 95% of children will produce antibodies and 70-90% will be protected against infection for at least 7-10 years after vaccination. According to Japanese researchers (Japan is the first country in which the vaccine was registered), immunity lasts 10-20 years. After studying an outbreak of chickenpox in one of the day hospitals, the results showed that the effectiveness of vaccinations was 100% in preventing severe cases and 86% in preventing the disease itself. The attack rate among unvaccinated susceptible children was 88%.
It is safe to say that the circulating varicella zoster virus promotes “re-vaccination” of vaccinated people, increasing the duration of immunity. After one dose of vaccines, seroconversion occurs in approximately 95% of healthy children.
Research suggests that emergency vaccination - when the vaccine is given within 72-96 hours of exposure to VZV - can also be effective - at least 90% protective efficacy can be expected. Chickenpox in people who have received the vaccine is much milder than in people who have not been vaccinated.
Vaccines used for chickenpox vaccination
The article analyzes the incidence of chickenpox at the present stage, and the development of a system for the prevention of this infection in the world. It is indicated that in Russia there is currently no domestic vaccine against chickenpox. Information is provided on the production of the Oka vaccine strain for the first time in Japan, on the development of vaccines and quality control requirements in Japan and Belgium. The results of assessing the quality of the Okavax and Varilrix vaccines when carried out at the GISC named after. L.A. Tarasevich laboratory pre-registration tests. An analysis of clinical trials of the above vaccines against chickenpox is given based on the materials presented in the drug dossier.
Chickenpox (chickenpox) is an acute and highly contagious disease caused by type 3 herpesvirus - Varicella zoster, belonging to the Herpesviridae (herpesvirus) family. Chickenpox (primary chickenpox) is considered a minor childhood infection; it affects mainly children from twelve months of age to 15 years. Children of older age groups may experience serious post-vaccination complications, such as secondary bacterial infection and pneumonia.
In adults and adolescents, the infection is severe and is often accompanied by complications (pneumonia, encephalitis, hepatitis, etc.), the likelihood of which increases with age. In cases of immune disorders (leukemia, cancer, immunodeficiencies of various origins), infection caused by the varicella-zoster virus is especially severe, sometimes fatal.
During pregnancy, the infection is also severe and chickenpox in the first trimester of pregnancy can cause damage to the central nervous system of the fetus, defects in the development of limbs, microophthalmia, cataracts and other eye diseases, including blindness.
After the illness, the virus enters the latent phase. When the infection is reactivated, most often at the age of 30 to 60 years and older as a result of various reasons (stress, concomitant respiratory infections, etc.), the virus, which usually persists in the spinal nerve root ganglia, is activated and a disease occurs - herpes zoster , occurring with severe pain, often leading to loss of ability to work. If left untreated, relapses of herpes zoster can occur repeatedly throughout life.
As with a number of other viral infections, such as measles, mumps, rubella, one of the leading means of protection against chickenpox is the chickenpox vaccine.
Currently, vaccination of children against chickenpox is included in the vaccination schedule of a number of European Union countries, including Germany, Great Britain, Italy, Spain, and France. The chickenpox vaccine Varilrix is registered in a number of CIS and Baltic countries - Ukraine, Kazakhstan, Azerbaijan, Uzbekistan, Estonia, Latvia, Lithuania.
In the USA, prevention against chickenpox has been carried out since 1995. Vaccination of children at risk among adolescents and adults has proven high clinical and economic effectiveness - since 1999 in the USA there has been a sharp decrease in morbidity and mortality from this disease.
The Okavax chickenpox vaccine has been introduced into the vaccination schedule in Japan, South America, Canada, Australia, South Korea, Taiwan and other Pacific countries. According to the results of prospective observation, the duration of post-vaccination immunity ranges from 6 to 11 years, in some cases up to 20 years.
In Russia, vaccination against chickenpox was not used until recent years (before foreign vaccines against chickenpox were registered in the Russian Federation), mandatory vaccination of children against chickenpox was not included in the Vaccination Calendar.
Between 2005 and 2008, two vaccines against chickenpox were registered in Russia - Varilrix, produced in Belgium and Okavax, produced by the Infectious Diseases Research Foundation of Osaka University (BI-KEN), Japan.
Laboratory control of batches, examination of submitted regulatory documents and reports on clinical trials of these vaccines, preparation of vaccines for registration in the Russian Federation were carried out at the Federal State Budgetary Institution GISC named after. L. A. Tarasevich Rospotrebnadzor.
Two more vaccines are being produced abroad: Varivax and Zostervax, produced in the USA, but these drugs were not submitted for registration in the Russian Federation.
In Belgium, the tetravaccine “MMRV” was developed and tested - a combined live attenuated vaccine against measles, mumps, rubella and chickenpox, but this drug was not submitted for registration in the Russian Federation. There is still no domestically produced vaccine against chickenpox in the Russian Federation.
The first research into the development of a vaccine against chickenpox began in Japan and consisted of creating a cultured live vaccine technology based on an attenuated (weakened) varicella zoster virus.
The Oka vaccine strain of varicella zoster virus was isolated in Japan in the 70s from the vesicular fluid of a boy named Oka who had a mild case of chickenpox. The virus was isolated and repeatedly passaged in a culture of human embryonic lung cells, then in a culture of guinea pig embryonic cells and in a culture of continuous human diploid WI-38 cells, and then in a culture of human diploid MRC-5 cells.
The virus obtained as a result of passages differed from wild strains of the varicella virus in a number of indicators—attenuation markers:
A. low level of reproduction in cell culture at a temperature of 390 ° C; b. a more pronounced immune response when immunizing guinea pigs compared to immunization with a wild virus; c. higher reproduction in cell culture of guinea pig embryos compared to wild strains of the varicella eoster;g virus. Restriction analysis revealed differences in the nucleotide composition of the genome of the Oka vaccine strain compared to the wild varicella zoster virus; d. lack of neurovirulence for monkeys sensitive to the varicella virus in the Oka vaccine strain (according to clinical and histological examination of brain-infected monkeys).
To maintain the strain, a system of seed series was used on the MRC-5 transplantable cell culture: vaccine strain (initial), working virus bank and seed virus - no more than 3-4 passages until a semi-finished vaccine was obtained.
Later, the Oka vaccine strain was transferred to a US scientific laboratory, where the virus was passaged in a human diploid cell culture - WI-38, and further passage of the virus was carried out in a MRC-5 cell culture. This is how the Varivax vaccine was created.
In Belgium, for the production of the Varilrix vaccine, the Oka strain was obtained under license from Osaka University, Japan; further development of the strain and production of the vaccine was carried out on the MRC-5 cell substrate.
In 1984, the WHO Committee of Experts on Biological Standardization, based on the results of studying the vaccine strain “Oka”, the development of technology for obtaining and methods of control of cultured live attenuated vaccine against chickenpox in various countries, developed international requirements for the vaccine strain of the varicella zoster virus “Oka” and vaccine production technologies, properties and control methods of the vaccine are determined.
International requirements for varicella vaccine, approved in 1985, were last revised by WHO in 1993 (WHO Technical Report Series 848, 1994). The attenuated strain “Oka” of the varicella zoster virus has been recognized as a vaccine for the production of vaccines against chickenpox in various countries around the world.
In accordance with materials presented by Belgium and the Osaka University Infectious Diseases Research Foundation, Japan, the Oka vaccine strain and a production culture of human diploid cells, MRC-5, were studied at all levels of passaging. When obtaining a production line of MRC-5 cell culture, sterility, absence of mycoplasmas and other foreign agents (on animal cell cultures and chicken embryos), absence of tumorigenicity, proliferative activity, karyology and identity were studied at each passage.
In the same way, the Oka strain was studied for sterility, the absence of mycoplasmas and other foreign agents, biological activity, authenticity, specific safety (lack of neurovirulence for monkeys sensitive to the varicella zoster virus), acute and chronic toxicity when infecting laboratory animals of various ages (mice , guinea pigs, white rats, rabbits).
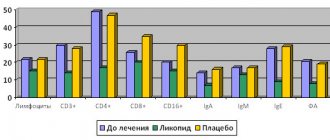
During the research process, the optimal composition of drugs per one vaccination dose was developed for both vaccines, which is given in Table. 1.
Table 1. Composition of the Varilrix and Okavax vaccines intended for the prevention of chickenpox
| "Varilrix" | "Okavax" |
| Live attenuated Varicella Zoster virus strain Oka - ≥ 103.3 PFU | Live attenuated Varicella Zoster virus strain Oka - ≥ 103.3 PFU |
| Human serum albumin – 1 mg | Buffer system:
|
| Lactose 32 mg | Sucrose 25 mg |
| Sorbitol 6 mg | |
| Mannitol 8 mg | Sodium glutamate 36 mg |
| Amino acids 8 mg | |
Antibiotic:
| Antibiotics:
|
The solvent for both vaccines is water for injection, 0.5 ml.
Based on the analysis and examination of materials on vaccine production technology, the results of preclinical tests presented by the manufacturers of both vaccines, the GISC named after. L. A. Tarasevich, the requirements for assessing the quality of vaccine series that were included in Russian regulatory documentation were edited and agreed upon. These requirements were used when carrying out laboratory testing of batches presented by the companies - and Okavax at the Federal State Institution GISC named after. L. A. Tarasevich Rospotrebnadzor (Table 2).
Table 2. Requirements and quality indicators of the Okavax and Varilrix vaccines
| ND indicators | Okavax standards | Varilrix standards |
| Description | Amorphous mass or white powder | Amorphous mass of creamy yellow color |
| Authenticity | The attenuated strain of the Varicella Zoster virus (Oka strain) contained in the vaccine must be neutralized by immune serum to the Varicella Zoster virus in a neutralization reaction in a culture of human diploid cells LEC, or any other culture sensitive to the varicella zoster virus; the presence of neutralization is detected by the method of indirect immunofluorescence by specific luminescence in cells infected with the varicella zoster vaccine virus - OKA strain. | The attenuated strain of the Varicella Zoster virus (Oka strain) contained in the vaccine must be neutralized by immune serum to the Varicella Zoster virus in a neutralization reaction in a culture of diploid human cells LEC, or any other culture sensitive to the varicella zoster virus. The neutralization index must be at least 1.2 lg. |
| Dissolution time | No more than 2 minutes. | No more than 2 minutes. |
| Description of the reconstituted drug | Colorless transparent or slightly whitish liquid | Transparent liquid from yellow-pink to pink |
| Transparency of the reconstituted drug | The opalescence of the solution must be comparable with standard II | The opalescence of the solution must be comparable with standard I |
| pH of the reconstituted drug | From 6.8 to 8.0 | From 6.9 to 7.4 |
| Mechanical inclusions of the reconstituted drug | There should be no visible inclusions | There should be no visible inclusions |
| Weight loss on drying | No more than 3.0% | No more than 3.0% |
| Pyrogenicity | Must be pyrogen-free | Must be pyrogen-free |
| Bacterial endotoxins | No more than 25 U/ml | No more than 25 U/ml |
| Abnormal toxicity | Must be non-toxic | Must be non-toxic |
| Sterility and absence of mycoplasmas | Must be sterile and free of mycoplasmas | Must be sterile and free of mycoplasmas |
Quantitative determination of antibiotics
| Not more than 7 mcg/0.5 ml Not more than 2 mcg/0.5 ml | No more than 25 mcg/dose |
| ||
| Bovine serum albumin (BSA) | Not more than 0.5 mcg/0.5 ml | No more than 0.5 mcg/dose |
| Specific activity | Contents of the Varicella Zoster vaccine virus; Oka strain, one vaccination dose (0.5 ml) must contain at least 1000 PFU of virus | The content of the Varicella Zoster vaccine virus, Oka strain, in one vaccination dose (0.5 ml) must be at least 1000 PFU of the virus |
In accordance with the requirements and descriptions of control methods, during laboratory control of three batches of each drug, it was established that their quality corresponded to the regulated indicators. The results of monitoring batches of the Okavax vaccine and batches of the Varilrix vaccine indicate that the vaccine batches comply with the requirements specified in Table. 2.
According to materials presented by the company, clinical trials of this drug were first conducted from 1973 to 1983, and 53 medical institutions in Japan participated in them. In accordance with international requirements, trials of the Okavax vaccine were carried out in three phases. During phase 1 trials, 2 batches of the vaccine were used for study.
The following population groups were used as contingents for vaccination:
- healthy children and adults;
- children with concomitant diseases;
- children and adults at high risk of infection;
When organizing the tests, the test rules in force in international practice were used:
- informed consent of adults or parents of children for vaccination;
- assessment of adverse reactions: local within 48 hours after vaccination, general within 4-6 weeks. after vaccination;
- isolation of the virus from vesicles if the latter appear;
- monitoring of persons who have been in contact with vaccinated persons in order to determine the possibility of infection of contact persons;
- immunological effectiveness in terms of seroconversion - assessment of the level of specific antibodies in the sera of those vaccinated in the RTGA, immunofluorescence and in the neutralization reaction;
- long-term (from 6 months to 7 years) observation of vaccinated people - study of the duration of immunity, chickenpox disease among those vaccinated with the vaccine.
As a result of the first phase of clinical trials, the following was established.
- In 41 vaccinated healthy children aged 2 to 5 years, specific antibodies were detected in more than 90% of those vaccinated, regardless of the dose of the virus in the vaccine (500 PFU, 200 PFU, 100 PFU); There were no side effects in healthy children during vaccination.
- Children with concomitant diseases: nephrosis, nephritis, thrombopenic purpura, osteomyelitis, hepatitis, enteritis, arthritis, bronchial asthma, meningitis, hemangioma - a total of 28 children, of which 23 were sero-negative, 12 received steroid therapy. The children were vaccinated due to contact with a child with chickenpox. The seroconversion rate was 100%, side effects: in 6 out of 28 - temperature above 370 C, vesicular rash in 2 children within 1-1.5 days after vaccination.
- 4 children aged 2 to 5 years were vaccinated, diagnosed with acute leukemia in the stage of complete remission, without chemotherapy for 2 weeks. before and 2 weeks after vaccination. The vaccine dose is 500 PFU. In all children, a fourfold or more increase in antibody titers was observed; fever and specific rashes were absent.
- When 30 healthy children were vaccinated with different doses of the vaccine virus (from 200 PFU to 2000 PFU), the production of antibodies and the absence of side effects were observed. When vaccinating 20 children with acute leukemia (from 1 year to 9 years), seroconversion was observed in 17 out of 20 people, side effects were manifested by fever and a mild rash in 50% of those vaccinated.
In phase 2 of the clinical trials of the Okavax vaccine, two series of the vaccine were used, obtained after 6 additional passages in the culture of diploid WI-38 cells. Children diagnosed with leukemia or other malignant neoplasms were vaccinated. The test conditions were the same as in phase 1, but not only vaccinated children were monitored, but also other family members.
- Acute leukemia. 102 children were vaccinated. Serocon version is from 88.9% to 93.3%, the frequency of adverse reactions is 18.1%.
- Solid malignant tumors. 13 children were vaccinated, the seroconversion rate was 92.3%, and an adverse reaction occurred in 1 child.
- Concomitant diseases (without a febrile component) 104 children were vaccinated. Seroconversion was 100%.
During phase 3 clinical trials, vaccinations were carried out using 18 batches of the vaccine obtained under production conditions on a culture of human diploid cells MRC-5. The purpose of phase 3 trials is to study the vaccine in practical use for patients at high risk of infection (leukemia, malignant tumors), nephrosis, collagenosis, bronchial asthma, and also when vaccinating adults - students, doctors, women (to prevent the occurrence of infection during pregnancy).
As a result of the phase 3 trials of the Okavax vaccine, 1,252 healthy children were vaccinated. Seroconversion was observed in 1206 children (96%), adverse reactions (mild) in 15 children (1%), and mild chickenpox was diagnosed in 13 children (1%) during one year of follow-up after vaccination.
2179 patients diagnosed with acute leukemia (on cessation of chemotherapy) were vaccinated. Seroconversion was determined in 166 people (93%). Adverse reactions: rash, mild fever in 31 people (17%), clinical manifestations of chickenpox were detected in 32 patients (18%) after vaccination (23 - mild, 7 - moderate, 2 - severe). The last two children with severe chickenpox were infected during a course of intensive chemotherapy.
333 patients with various concomitant diseases were vaccinated. Seroconversion in 306 patients (92%), adverse reactions in 15 people (5%), chickenpox was diagnosed in 9 vaccinated people (3%). Long-term observations of vaccinated people were carried out for 7 years.
A total of 810 patients were observed (neurological diseases - 276, heart diseases - 146, malignant neoplasms - 87, immunological and allergic diseases - 155, congenital anomalies and malformations - 78, diseases of the gastrointestinal tract and endocrine system - 68), of which Of these, only 21 patients showed symptoms of chickenpox after vaccination, and in 4 cases the development of herpes zoster was noted.
Currently, the Okavax vaccine is used in 32 countries around the world. During the period from 2004 to 2007, the BIKEN enterprise produced and sold more than 4.5 million doses of the Okavax vaccine in Japan and other countries of the world; during this period, only 38 cases of adverse reactions of varying severity were recorded with the predominance of mild reactions.
The Okavax vaccine is intended for:
- for the prevention of chickenpox from 12 months, primarily in persons classified as high-risk groups who have not had chickenpox and have not been vaccinated previously.
- for emergency prevention of chickenpox in persons who have not had chickenpox and have not been vaccinated previously, who were in close contact with patients with chickenpox (family members, doctors, paramedical and junior medical personnel, as well as other persons). Vaccination schedules:
- children from 12 months to 13 years - 1 dose (0.5 ml) of the vaccine once, subcutaneously.
- persons over 13 years of age (including those in contact with high-risk groups and sick people) - 1 dose (0.5 ml) twice with an interval between doses of 6-10 weeks, subcutaneously.
Emergency prophylaxis - 1 dose (0.5 ml), once, subcutaneously within the first 96 hours after contact (preferably within the first 72 hours). The Varilrix vaccine was studied as part of an international trial: “A blinded, randomized, controlled, multicenter study of a live attenuated varicella vaccine produced in Belgium, administered in a single dose regimen, and a combination vaccine against measles, mumps, rubella, chickenpox, produced in . Belgium, prescribed in a two-dose regimen to healthy children during the second year of life.” Trials were conducted to evaluate clinical effectiveness against chickenpox disease.
Clinical trials of the Varilrix vaccine as part of the above trial were conducted in the period 2005-2006. The immunological effectiveness (% of seroconversions and GMT (geometric mean titer) of antibodies to the varicella zoster virus) and the safety of the vaccine were assessed 42 days, 84 days, 1 year, 2 years after a single vaccination of children of the second year of life.
In this international study, children were vaccinated with the Varilrix vaccine also in Russia. In total, more than 5,000 children were vaccinated, of which 397 children aged 12 to 22 months were vaccinated in Russia, who had no contraindications to vaccination, who had not had chickenpox, and whose parents signed informed consent to participate in this study. During a two-year observation of vaccinated children in Russia, not a single case of chickenpox was identified.
In general, with a single vaccination, 42 days after vaccination, seroconversion was observed in 97% of those vaccinated, in 55% of children, the antibody GST was more than 1:128, while low reactogenicity rates were detected - 20% of moderate local reactions, 3.8% of moderate temperature reactions reactions in the complete absence of serious adverse events.
Based on the certification of the series of vaccines against chickenpox, Okavax and Varilrix, for compliance with the requirements of regulatory documentation, analysis of the results of preclinical studies and international clinical trials conducted, organized by, many years of use of vaccines in various countries of the world - the vaccine against chickenpox "Varilrix" and "Okavax" - registered in the Russian Federation and recommended for practical use for vaccination of children from twelve months of age and adults.
The current situation with chickenpox in Russia is more than 1000 cases of chickenpox per 100 thousand population, the incidence of children has almost doubled in recent years, and severe damage is caused to the health of adults, especially the elderly.
Among airborne infections (excluding influenza and ARVI), chicken pox accounts for 50-70%. Every year in the Russian Federation, 500-800 thousand cases of this disease are registered, while 10 thousand children develop severe complications, about 50 people die annually. Mostly children aged 3-6 years are affected; the contagiousness index reaches 100%.
The current situation urgently requires organizational measures to prevent chickenpox in Russia. It is necessary, taking into account the accumulated international experience, to consider the possibility of organizing domestic production of the chickenpox vaccine to provide the population of the Russian Federation with the required number of vaccine doses.
Literature:
- Vorobyova M. S. Ladyzhenskaya I. P., Barkhaleva O. A. - “Current state of vaccine prevention of chickenpox (Varicella zoster)” Abstracts of the All-Russian scientific and practical conference “Vaccinology 2008. Improving immunobiological means of prevention, diagnosis and treatment of infectious diseases”, Moscow, 2008, p. 32.
- VC. Tatochenko - Vaccination against chickenpox. We answer questions from pediatricians (reprint) - J. Questions of modern pediatrics, 2009, No. 3 (vol. 8).
- “Treatment and prevention of chickenpox in children in modern conditions” Methodological recommendations prepared by Timchenko V.N. with co-authors, St. Petersburg, 2008, 32 pages. Infectious morbidity in the Russian Federation in 2005-2006 (Information collection) / Federal Center for State Sanitary and Epidemiological Sciences of the Ministry of Health of the Russian Federation, M. 2007, pp. -5, 13, 20.
- Instructions for use of the Varilrix vaccine.
- Instructions for use of the Okavax vaccine.
Post-vaccination reactions
The chickenpox vaccine is very easily tolerated by people; reactions to it are very rare. The majority of people who experienced any reactions to the vaccine developed local manifestations. Local reactions include the following symptoms at the injection site: swelling, induration, redness and slight soreness. These symptoms disappear within a few days and develop on the first day after immunization. In addition to local reactions, general symptoms develop in 0.1-5% of cases. Common reactions to the chickenpox vaccine include fever, rash, itchy skin, weakness, fever, and enlarged and painful lymph nodes.
What risks are associated with the chickenpox vaccine?
A vaccine, like any medicine, can cause serious problems, such as severe allergic reactions. However, the risk that vaccination will cause serious harm or death is negligible.
The chickenpox vaccine is much safer than getting chickenpox. For most people, the chickenpox vaccine does not cause any complications. In general, reactions are more likely to occur after the first dose than after the second.
Minor complications
- Soreness or swelling at the injection site (affects about 1 in 5 children and 1 in 3 teenagers and adults).
- Fever (1 in 10 people or less).
- Mild rash within 1 month after vaccination (1 in 25 people). It is possible that vaccinated people could infect other family members, but this is extremely rare.
Moderate violations
- Convulsions (twitching or fixed gaze) caused by high fever (extremely rare).
Severe violations
- Pneumonia (extremely rare)
Other serious problems have been reported following chickenpox vaccination, including severe brain reactions and low blood cell levels. They are so rare that experts find it difficult to say whether they were caused by the vaccine. If yes, then this happens extremely rarely.
Note. The first dose of MMRV vaccine causes a rash and a higher fever than MMR and chickenpox vaccine when given separately. A rash occurred in about 1 in 20 people, and a fever in about 1 in 5 people. Seizures caused by fever also occurred more frequently after MMRV was given. They usually occur 5 to 12 days after the first dose.
Contraindications
Chickenpox vaccination is contraindicated for people who at that time have acute illnesses or exacerbation of chronic pathology. In this case, the vaccine is given after recovery or after achieving stable remission.
If a person has had mild respiratory or intestinal infections, then the vaccine can be given after 2-4 weeks. If there were severe diseases of the nervous system (for example, meningitis), then the vaccination can be done at least six months after recovery.
Vaccination is absolutely contraindicated in severe immunodeficiency, when the number of lymphocytes in the blood is less than 1200 per ml. Such severe immunodeficiency can be observed against the background of tumor pathologies, AIDS, the use of corticosteroids, etc.
Pregnancy (or planned pregnancy within 3 months) is also a contraindication for chickenpox vaccination. If surgery is to be performed, the vaccination should be done at least a month before the procedure.
Vaccines have another contraindication - an allergy to neomycin or other components of the drug, as well as a severe reaction to a previous vaccine administration.
You cannot vaccinate against chickenpox if there have been episodes of administration of immunoglobulins and blood products within six months prior to this point. You should refrain from administering these drugs for 3 weeks after the chickenpox vaccination.
Why is vaccination necessary?
Vaccination is performed to produce antibodies to the third type of herpes virus, which causes the disease. Thanks to this, the human body will be able to resist pathogens even when in contact with an infected person.
The vaccine is not included in the national vaccination schedule. Therefore it is not free. However, it is recommended for everyone to do it. The positive result that a person receives from it pays for the cost. Vaccination is recommended for employees of kindergartens and schools. Chickenpox outbreaks are more likely to occur in these facilities.
Adverse reactions
From the second to the third week after vaccination, delayed general reactions may appear; there is no need for special treatment, they go away on their own:
- slight increase in temperature;
- general malaise;
- rare rashes resembling chickenpox, accompanied by itching;
- enlarged lymph nodes.
Side effects:
- herpes zoster;
- brain inflammation;
- deterioration of sensitivity;
- decreased platelet levels;
- pain in large joints.
According to WHO, such manifestations occur in fifteen out of one million people. If you experience unfavorable symptoms, you should consult a doctor for help.
Vaccination validity period
Immunity to herpes virus type 3 is formed 6 weeks after administration of the drug. However, vaccination cannot provide a 100% guarantee that infection with a pathogen will not occur. Vaccination reduces the likelihood of infection by 95-98% and the risk of chickenpox complications.
According to the manufacturing companies, the validity period of the drugs is 30 years. This was proven by studies conducted 20 years ago. Antigens to pathogenic microorganisms were found in vaccinated people. Therefore, vaccination is usually done 1-2 times throughout a lifetime.
Possible side effects
Local reactions occur rarely: pain and redness at the injection site.
General reactions occur extremely rarely: increased body temperature to low-grade levels (rectal ≥ 38°C, in the armpit or oral cavity: ≥ 37.5°C), lymphadenopathy, weakness, malaise. A rash similar to chickenpox may appear. The rash is not abundant and rarely develops into blisters. In isolated cases, absolutely extremely rarely, vaccinated people may develop fainting or clonic-tonic convulsions within 15 minutes after vaccination.
Varilrix should be used with caution in cases of thrombocytopenia or blood coagulation disorders, since bleeding may occur during intramuscular administration.
Come get vaccinated at VIRILIS. A full range of vaccines for children and adults, family vaccinations - at a special price!
Vaccine administration method
The vaccine is administered to a healthy patient by a medical professional with special clearance. A specialist passes exams annually to obtain it. It is impossible to vaccinate yourself on an outpatient basis.
The drug must be in the refrigerator and not expired. The injection kit includes a dry substance with an active element - lyophilisate and purified water. The solution is prepared in a dark room. Exposure to ultraviolet rays is unacceptable. The drug should not be stored in already prepared form.
The injection is administered under the skin. Most often, the injection is placed in the deltoid muscle of the shoulder. This reduces the likelihood of adverse reactions. The vaccine can also be injected under the shoulder blade, in the outer part of the thigh. The medication is not used intravenously.
The patient spends half an hour in the hospital after the event. Doctors monitor the manifestations of allergic reactions and give recommendations on the regimen during the post-vaccination period.
Compatibility with other vaccines
The Varilrix vaccine can be administered simultaneously with all drugs from the national schedule of preventive vaccinations on the same day, in different parts of the body, with the exception of the BCG vaccine and rabies vaccine. The use of the Varilrix vaccine together with other vaccinations does not affect their immunogenicity (ability to develop immunity). Tolerability of vaccines does not deteriorate, and the number of adverse reactions does not increase. Administering several vaccines on the same day does not place an excessive burden on the immune system.
Varilrix should not be given concomitantly with other live attenuated vaccines, with the exception of the combined measles, rubella and mumps vaccine. However, if these vaccines were not prescribed at the same time, then the interval between their administration to achieve maximum antibody levels should be at least 30 days.
Administration of the Varilrix vaccine is possible no earlier than 3 months after the administration of immunoglobulins or after a blood transfusion (blood transfusion).
If it is necessary to perform a tuberculin test (Mantoux), it should be performed before vaccination, since according to available data, live viral vaccines can cause a temporary decrease in skin sensitivity to tuberculin. Due to the fact that such a decrease in sensitivity can persist for up to 6 weeks, tuberculin testing should not be performed within the specified period of time after vaccination to avoid obtaining false negative results.