There are many causes for hypocalcemia and hyperphosphatemia. But secondary hyperparathyroidism, which develops in patients receiving renal replacement therapy, deserves special attention. A progressive decrease in the number of functioning nephrons in chronic renal failure causes disruption of all links in the regulation of phosphorus-calcium metabolism.
When hyperphosphatemia occurs, there is a response decrease in ionized calcium. Hyperphosphatemia and hypocalcemia directly stimulate the synthesis of parathyroid hormone (PTH) by the parathyroid glands. Calcium affects the processes of PTH synthesis through calcium receptors present in the parathyroid glands, the number and sensitivity of which decrease. As the decline in renal function progresses, a deficiency of calcitriol, the active metabolite of vitamin D3 synthesized in the kidneys, also occurs, and the number of calcitriol receptors in the parathyroid glands decreases. As a result of these processes, the inhibitory effect of calcitriol on the synthesis and secretion of PTH is weakened, and skeletal resistance to the calcemic effect occurs, which is also accompanied by hypersecretion of PTH.
Symptoms of hyperparathyroidism
Initially, with the development of hyperparathyroidism, patients feel nothing but general weakness. However, as the disease progresses, symptoms such as:
- pain in bones and joints;
- muscle weakness;
- fatigue;
- decreased tone;
- fractures;
- manifestations of urolithiasis, due to excessive load on the kidneys due to the need for increased excretion of calcium from the body;
- gastrointestinal disorders due to mineral disturbances and changes in appetite;
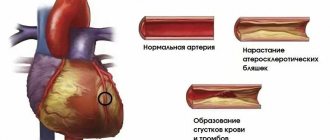
- increased atherosclerotic changes due to calcium deposits in the walls of blood vessels;
- cardiac dysfunction;
- manifestations of peptic ulcer disease due to impaired blood supply to the walls of the mucous membrane of various parts of the gastrointestinal tract and the formation of peptic ulcers;
- psychoneurological disorders due to metabolic disorders of nerve fibers.
How does this happen?
As a result of prolonged and uncontrolled effects of PTH on osteoclasts, hyperplasia (an increase in the number of cells) and activation of the latter occurs. Due to the uncontrolled activation of osteoclasts, the same uncontrolled destruction of bone tissue and proliferation of connective tissue occurs. Often this process takes on a cystic-fibrous character: bone tissue is replaced by closed cavities, among which there is an accumulation of cells (osteoclasts, fibroblasts) and connective tissue fibers.
Classification
Primary hyperparathyroidism
Occurs in patients with a tumor of the parathyroid glands (adenoma), which microscopically represents a genetically similar focal growth of tissue; usually only one parathyroid gland is affected.
Secondary hyperparathyroidism
develops as a response to a long-term decrease in the level of Ca2+ in the blood due to vitamin D deficiency, manifestations of chronic renal failure or severe absorption disorders in the gastrointestinal tract, as well as due to hemodialysis in patients. Microscopic changes. At this stage, regression of the disease is still possible with adequate correction of the causes that caused it, reverse development of morphological changes in the parathyroid glands and stabilization of the patient’s PTH level.
Tertiary hyperparathyroidism
occurs with long-term secondary hyperparathyroidism, with the formation of adenomas, amenable only to surgical correction.
The approach to the treatment of different forms of hyperparathyroidism, indications for surgery, as well as the scope of required surgical intervention differ significantly. Specialists at the Endocrine Surgery Clinic can determine your individual treatment plan after conducting the necessary diagnostic tests.
Publications in the media
Regarding the treatment of this disease, you can contact the Surgical Department No. 2 of the Clinic of Faculty Surgery named after. N.N. Burdenko
Hyperparathyroidism is a disease of the endocrine system caused by excessive secretion of PTH and characterized by severe disturbances in the metabolism of calcium and phosphorus. There are primary, secondary and tertiary hyperparathyroidism • Primary. Hyperfunction of the parathyroid glands due to their hyperplasia or neoplasm. Two thirds of cases of primary hyperparathyroidism occur in postmenopausal women • Secondary develops compensatoryly and is usually found in chronic renal failure in conditions of vitamin D deficiency and prolonged hyperphosphatemia or malabsorption syndrome in conditions of chronic hypocalcemia • Tertiary is caused by a developing adenoma of the parathyroid glands against the background of long-standing secondary hyperparathyroidism • Pseudohyperparathyroidism ( ectopic hyperparathyroidism) is observed in malignant tumors of various locations (bronchogenic cancer, breast cancer, etc.); associated with the ability of some malignant tumors to secrete PTH.
Statistical data. 1 case per 1000 population (primary hyperparathyroidism). Predominant age: 20–50 years. The predominant sex is female (2.5:1).
Genetic aspects • Familial primary hyperparathyroidism with multiple ossifying fibromas of the jaw (*145001, Â) • Familial primary hyperparathyroidism (*145000, Â) due to hyperplasia of the chief cells of the parathyroid glands • Congenital primary hyperparathyroidism with hypercalciuria (239199, r). Clinically: nephrocalcinosis, renal tubular acidosis, developmental delay, nausea. Laboratory: elevated PTH levels, hypercalcemia, hypercalciuria • Congenital familial hyperparathyroidism (#239200, r or Â) - the disease is caused by homozygosity for the mutant parathyroid calcium receptor gene, which in the heterozygous state causes familial hypocalciuric hypercalcemia (145980). Clinically: congenital primary hyperparathyroidism, developmental delay, decreased appetite, constipation, thirst, hepatomegaly, splenomegaly, polyuria, renal calcinosis, arterial hypotension, multiple fractures, metaphyseal abnormalities, osteoporosis, narrow chest, shortness of breath, anemia. Laboratory: hypercalcemia, hypophosphatemia, hypercalciuria, hyperphosphaturia, aminoaciduria, increased levels of parathyroid hormone in the blood serum, hyperplasia of parathyroid cells.
Risk factors • Age over 50 years • Female gender • Ionizing radiation.
Etiology and pathogenesis • Primary hyperparathyroidism •• Single parathyroid adenoma - 80–90% of cases; hyperplasia of all four glands causes 10–20% of cases of primary hyperparathyroidism. Parathyroid cancer is rare •• PTH increases blood calcium levels by stimulating the formation of vitamin D and its conversion to calcitriol (important for calcium absorption in the gastrointestinal tract), increasing renal tubular calcium reabsorption, decreasing renal tubular phosphate reabsorption, and mobilizing calcium from bones •• Elevated levels of circulating calcium ions inhibit PTH synthesis. It is believed that the parathyroid adenoma is capable of functioning autonomously, producing, despite a high level of serum calcium, an excessive amount of PTH and leading to primary hyperparathyroidism • Secondary hyperparathyroidism •• Most often observed in chronic renal failure with the development of adaptive hyperplasia and hyperfunction of the parathyroid glands due to prolonged hypocalcemia. Other causes: rickets, Fanconi syndrome, malabsorption syndrome •• Impaired production of calcitriol in the kidneys due to vitamin D deficiency leads to impaired absorption of calcium in the gastrointestinal tract and hypocalcemia •• Damage to the renal parenchyma leads to hyperphosphatemia •• Resistance of bone and renal tissue to PTH.
The clinical picture depends on the concentration of calcium in the blood serum. The disease usually manifests as mild asymptomatic hypercalcemia, although patients with classic signs of advanced kidney and bone damage are sometimes observed. When calcium levels exceed 11–12 mg%, neurological and gastrointestinal symptoms appear. At a content of 14–20 mg%, a hyperparathyroid hypercalcemic crisis develops, manifested by uncontrollable vomiting, thirst, muscle and joint pain, symptoms of an acute abdomen, an increase in body temperature to 40 ° C and impaired consciousness. Mortality is 50–60%. Manifestations of hyperparathyroidism itself: • Renal •• Hypercalciuria and urinary tract stones •• Chronic hypercalcemia leads to the deposition of calcium salts in the renal parenchyma (nephrocalcinosis), renal failure occurs •• Polyuria and thirst due to hypercalciuria with damage to the epithelium of the renal tubules and a decrease in the sensitivity of renal tubular receptors to ADH • Skeletal. Excess PTH increases bone resorption by osteoclasts and leads to a disorder of bone metabolism (parathyroid osteodystrophy, or von Recklinghausen's disease) • Gastrointestinal •• Anorexia •• Weight loss •• Constipation •• Nausea •• Vomiting •• Abdominal pain •• Ulcers are common illness and pancreatitis • Neurological and mental •• Emotional lability •• Intellectual impairment •• Fatigue •• Muscle weakness • Cardiovascular •• Arterial hypertension •• Shortening of the Q-T interval • Articular and periarticular •• Arthralgia •• Gout •• Pseudogout • Ophthalmic •• Keratopathy •• Conjunctivitis due to calcium deposition in the conjunctiva • Skin - itching.
Concomitant pathology. Familial polyendocrine adenomatosis.
Age characteristics. Older people are more likely to have secondary hyperparathyroidism.
Differential diagnosis • Tumors (see Hypercalcemia in Malignant Tumors) • Sarcoidosis causes hypercalcemia due to the production of calcitriol by granulomatous tissue. In the absence of elevated PTH levels and signs of sarcoidosis, ex juvantibus therapy is recommended. In most cases of sarcoidosis, a decrease in serum calcium levels is observed within 1 week after administration of GC (for example, 40 mg prednisolone daily), but this is not observed in primary hyperparathyroidism • Familial hypocalciuric hypercalcemia (*145980, 3q21–q24, mutation of the HHC1, FHH, Â). Mild to moderate hypercalcemia, complications (urinary stones and renal failure) are rare •• PTH levels are normal or slightly elevated, parathyroid hyperplasia is possible, but subtotal parathyroidectomy does not reduce hypercalcemia •• The key to diagnosis is the familial nature of the disease and low urinary calcium excretion •• Due to the ineffectiveness of surgical intervention, this condition should be excluded in patients with primary hyperparathyroidism • Hypervitaminosis D • Milk-alkali syndrome is caused by the consumption of large quantities of calcium and absorbable alkalis; characterized by hypercalcemia, systemic alkalosis and kidney damage due to nephrocalcinosis. The risk of this syndrome increases with daily intake of more than 5 g of calcium carbonate (or 2 g in terms of calcium), which is 2 times the dose recommended for the prevention or treatment of osteoporosis • Other causes of hypercalcemia •• Hyperthyroidism causes hypercalcemia due to increased bone turnover •• Thiazide diuretics reduce urinary calcium excretion but rarely cause hypercalcemia; they are not prescribed to patients with hyperparathyroidism •• Prolonged immobilization can lead to hypercalcemia due to bone resorption. The problem especially often occurs in children who are bedridden for a long time (for example, in the case of a corset in the treatment of multiple fractures) •• Osteitis deformans •• Recovery from acute renal failure caused by necrosis of skeletal muscles is often accompanied by hypercalcemia. During the first 2–3 days after muscle injury, local deposition of calcium and phosphate occurs due to the destruction of myocytes. When kidney function normalizes, calcium and phosphates leave the areas of muscle damage and enter the bloodstream, causing hypercalcemia. This disorder usually occurs 2–3 weeks after acute muscle injury.
Laboratory Findings • Hypercalcemia is the cardinal sign of primary hyperparathyroidism • Hypophosphatemia • Serum chloride to phosphate ratio is usually elevated (>33), which is explained by increased serum Cl– (due to PTH-induced bicarbonaturia) • Serum ALP activity is increased only in patients with significant bone loss pathology • Hypercalciuria, but due to the Ca2+-reabsorbing action of PTH, in approximately one third of patients the calcium content in the urine is normal • An increase in the level of PTH in the blood in the presence of hypercalcemia is a strong argument in favor of primary hyperparathyroidism, because for other reasons for increased calcium, a decrease in PTH is observed. However, in some cases, a negative test result does not definitively exclude the possibility of primary hyperparathyroidism.
The influence of drugs. Lithium preparations, thiazide diuretics or ergocalciferol may increase serum calcium levels.
Special studies • Ultrasound, CT, MRI and isotope scanning are non-invasive methods that detect parathyroid adenomas in 60-80% of cases • Selective venous catheterization - collect blood samples from veins draining various areas of the neck and mediastinum. A significant increase in PTH concentration makes it possible to determine the location of the adenoma. The procedure is performed on patients before re-operation after an unsuccessful revision of the neck area.
TREATMENT
Lead tactics. In patients over 50 years of age without clinical symptoms and with serum calcium levels no higher than 11.4–12 mg%, the disease often does not progress for 10 years or more. Patients are under observation; surgical treatment is recommended if calcium levels increase, the patient develops kidney damage, skeletal damage, or other manifestations characteristic of a severe course of the disease.
Surgical treatment is the only radical and effective method.
• Preoperative preparation •• Ergocalciferol 2-3 days before surgery •• In case of dehydration - intensive rehydration •• Sanitation of the urinary tract • Anesthesia - general anesthesia • Intraoperative tactics •• In most cases, a single adenoma is found to be removed •• Three glands are removed and part four •• Remove all tissue of the parathyroid glands with transplantation of part of the tissue into the muscles of the forearm •• In approximately 10% of cases, during initial revisions of the neck area, it is not possible to identify altered tissue of the parathyroid gland
• The postoperative period •• Usually proceeds relatively easily •• Patients recover within a few weeks.
• Postoperative complications •• Damage to the recurrent nerve •• Tetany •• AKI •• Acute pancreatitis •• Transient hypocalcemia •• “Hungry bone” syndrome.
Conservative treatment is indicated if there are contraindications to surgical treatment, the patient refuses surgery or is unsuccessful • Increased fluid intake and physical activity help reduce serum calcium levels • Phosphates in doses of 1–2 g per day orally. The main complication is extraosseous calcification (very rare) • Estrogens (postmenopausal replacement therapy) reduce mildly elevated serum calcium levels by decreasing bone resorption.
Emergency treatment of hyperparathyroid hypercalcemic crisis.
• Forced diuresis (up to 4 liters of 0.9% sodium chloride solution intravenously in combination with the administration of furosemide intravenously).
• Bisphosphonates (pamidronic and etidronic acids) cause a decrease in serum calcium levels within 1–7 days •• Pamidronic acid 60–90 mg IV once a day; if necessary, the administration is repeated after 7 days •• Etidronic acid 7.5 mg/kg IV over 4 hours 1 time per day for 3–7 days.
• Gallium nitrate 200 mg/m2/day as a long-term infusion for 5 days. Use is limited by potential nephrotoxicity.
• Calcitonin 4 IU/kg IM or SC every 12 hours (acts faster, more briefly, weaker than plicamycin, gallium nitrate and pamidronic acid, but less toxic).
• GCs do not affect serum calcium levels in patients with hyperparathyroidism.
Course and prognosis • The prognosis is extremely favorable for primary hyperparathyroidism after surgical treatment • The prognosis is not very favorable for secondary and tertiary hyperparathyroidism (determined by the functional state of the kidneys).
ICD-10 • E21 Hyperparathyroidism and other disorders of the parathyroid [parathyroid] gland
Diagnostics
Even before clinical manifestations, patients suffering from hyperparathyroidism experience an increase in calcium levels in a biochemical blood test, an increase in urine calcium levels, and an increase in blood PTH levels. Often, especially in postmenopausal women, insufficient levels of vitamin D in the blood are detected.
Densitometry (examination of bone tissue) reveals severe osteoporosis due to increased bone tissue breakdown under the influence of PTH.
To visually identify and determine the location of altered parathyroid glands, the following is performed:
- Ultrasound of the neck area
- Scintigraphy of the parathyroid glands, which allows you to determine the hyperfunction of the parathyroid glands and establish the form of the disease (primary or secondary).
Currently, puncture biopsy of the parathyroid glands is not performed, which is associated with the usually high density of the formation, often its small size (up to 1 cm) and the high risk of tumor disintegration and spread of parathyroid cells along the puncture channel, which contributes to the progression of the disease and complicates further surgical treatment .
The wide range of manifestations and variability of the course of the disease require comprehensive examination and treatment of patients by highly qualified specialists.
However, with already pronounced changes in calcium-calcium-phosphorus metabolism, even with minimal symptoms, the only option for productive treatment is surgical intervention.
Observation and forecast
The prognosis of such patients is determined by the success of correction of hyperparathyroidism. To date, there are no reliable data on long-term outcomes in such patients, but there is limited data indicating a high likelihood of regression of brown tumors after surgical treatment of primary hyperparathyroidism. For patients with secondary hyperparathyroidism due to CKD, the prognosis is also determined by the course of the underlying disease, and the treatment of brown tumors appears to be limited to drug treatment or kidney transplantation.
After hemiparathyroidectomy, as well as during drug treatment (zincalcet, zoledronic acid, vitamin D preparations), a dynamic assessment of laboratory parameters reflecting bone metabolism, in particular, PTH, blood calcium (total and ionized), alkaline phosphatase, phosphates is necessary. An acceptable method for assessing the therapeutic effect of hemiparathyroidectomy is PET/CT in the proven absence of a primary tumor, which can be a source of bone metastases, and subject to a PET/CT study before treatment.
It is noteworthy that the incidence of brown tumors has been decreasing over the past decades due to improved diagnostic methods and timely detection of hyperparathyroidism in patients. The extremely rare occurrence of brown tumors in the general population makes it difficult to create clear guidelines for the management of such patients, and in each specific case we are the subjects of a unique experiment with a limited amount of known data and treatment options. Nevertheless, in such conditions, the need for prevention and timely correction of hyperparathyroidism is obvious as the only possible method of combating brown tumors.
Bibliography:
- Philip Brabyn et al. HYPERPARATHYROIDISM DIAGNOSED DUE TO BROWN TUMORS OF THE JAW: A CASE REPORT AND LITERATURE REVIEW. Journal of Oral and Maxillofacial Surgery, 2021 DOI: 10.1016/j.joms.2017.03.013
- Modern ideas about the action of thyroid hormones and thyroid-stimulating hormone on bone tissue / Zh. E. Belaya, L. Ya. Rozhinskaya, G. A. Melnichenko. Problems of endocrinology, Vol. 52, No. 2 (2006). https://doi.org/10.14341/probl200652248-54
- Manolagas S. S // Endocr. Rev. - 2000. - Vol. 21. — P. 115-137
- 引用本文:方义杰, 洪国斌, 卢慧芳, 等. 棕色瘤的临床病理特征及影像学表现(Clinicopathological features and imaging manifestations of brown tumors) [J]. 中华医学杂志,2015,95 (45): 3691- DOI: 10.3760/cma.j.issn.0376-2491.2015.45.012
- Queiroz, IV, Queiroz, SP, Medeiros, R. et al. Brown tumor of secondary hyperparathyroidism: surgical approach and clinical outcome. Oral Maxillofac Surg 20, 435–439 (2016). https://doi.org/10.1007/s10006-016-0575-0
- Endocrine pathology / M. Kettyle William, A. Arky Ronald, p.145 – 151, 2016
- Levine MR, Chu A, Abdul-Karim FW. Brown Tumor and Secondary Hyperparathyroidism. Arch Ophthalmol. 1991;109(6):847–849. doi:10.1001/archopht.1991.01080060111036
- Santini-Araujo et al. (eds.), Tumors and Tumor-Like Lesions of Bone: For Surgical Pathologists, 815 Orthopedic Surgeons and Radiologists, DOI 10.1007/978-1-4471-6578-1_59, © Springer-Verlag London 2015
- Surgical approach and clinical outcome of a deforming brown tumor at the maxilla in a patient with secondary hyperparathyroidism due to chronic renal failure. Arquivos Brasileiros de Endocrinologia & Metabologia On-line version ISSN 1677-9487 Arq Bras Endocrinol Metab vol.50 no.5 São Paulo Oct. 2006 https://doi.org/10.1590/S0004-27302006000500021
- Nicola Di Daniele, Stefano Condò, Michele Ferrannini, Marta Bertoli, Valentina Rovella, Laura Di Renzo, Antonino De Lorenzo, “Brown Tumor in a Patient with Secondary Hyperparathyroidism Resistant to Medical Therapy: Case Report on Successful Treatment after Subtotal Parathyroidectomy,” International Journal of Endocrinology, vol. 2009, Article ID 827652, 3 pages, 2009. https://doi.org/10.1155/2009/827652
- W. J. Marshall. Clinical biochemistry, 6th edition p. 239 – 255
- HELL. Taganovich et al. Pathological biochemistry, pp. 282 – 316, 2015
- Sager, Sait et al. “Positron emission tomography/computed tomography imaging of brown tumors mimicking multiple skeletal metastases in patient with primary hyperparathyroidism.” Indian journal of endocrinology and metabolism vol. 16.5 (2012): 850-2. doi:10.4103/2230-8210.100682
- Jaime Alonso Reséndiz‐Colosia et all, Evolution of maxillofacial brown tumors after parathyroidectomy in primary hyperparathyroidism. J.of the sciences and specialties of the head and neck, vol. 30, issue 11, pages 1497 – 1503, 2016
- Highly Aggressive Brown Tumor in the Jaw Associated with Tertiary Hyperparathyroidism. Authors: Pinto, Lecio Pitombeira; Cherubinim, Karen; Salum, Fernanda Gonçalves; Yurgel, Liliane Soares; De Figueiredo, Maria Antonia Zancanaro. Source: Pediatric Dentistry, Volume 28, Number 6, November/December 2006, pp. 543-546(4)
- Tatiana Clementino Pinto Toscano de França et all. Bisphosphonates can reduce bone hunger after parathyroidectomy in patients with primary hyperparathyroidism and osteitis fibrosa cystica. Revista Brasileira de Reumatologia. Print version ISSN 0482-5004 Rev. Bras. Reumatol. vol.51 no.2 São Paulo Mar./Apr. 2011 https://doi.org/10.1590/S0482-50042011000200003
- L Darryl Quarles et al. Management of secondary hyperparathyroidism in adult dialysis patients, UpToDate, Mar 15, 2021
- Effect of alfacalcidol on the natural course of renal bone disease in mild to moderate renal failure. Hamdy NA, Kanis JA, Beneton MN, Brown CB, Juttmann JR, Jordans JG, Josse S, Meyrier A, Lins RL, Fairey IT BMJ. 1995;310(6976):358.
- Bisphosphonate pathway, and the genes are involved in the effects of bisphosphonates on osteoclasts. © PharmGKB. Reproduced with permission from PharmGKB and Stanford University. Gong Li, Altman Russ B, Klein Te. Bisphosphonates pathway. Pharmacogenetics and genomics (2009)
- Structure-Activity Relationships for Inhibition of Farnesyl Diphosphate Synthase in Vitro and Inhibition of Bone Resorption in Vivo by Nitrogen-Containing Bisphosphonates. James E. Dunford, Keith Thompson, Fraser P. Coxon, Steven P. Luckman, Frederick M. Hahn, C. Dale Poulter, Frank H. Ebetino and Michael J. Rogers. Journal of Pharmacology and Experimental Therapeutics February 2001, 296 (2) 235-242;
- Wetmore, James B et al. “A Randomized Trial of Cinacalcet versus Vitamin D Analogues as Monotherapy in Secondary Hyperparathyroidism (PARADIGM).” Clinical journal of the American Society of Nephrology: CJASN vol. 10.6 (2015): 1031-40. doi:10.2215/CJN.07050714
- Wang, G., Liu, H., Wang, C. et al. Cinacalcet versus Placebo for secondary hyperparathyroidism in chronic kidney disease patients: a meta-analysis of randomized controlled trials and trial sequential analysis. Sci Rep 8, 3111 (2018). https://doi.org/10.1038/s41598-018-21397-8
- Zekri, Jamal et al. “The anti-tumour effects of zoledronic acid.” Journal of bone oncology vol. 3.1 25-35. 15 Jan. 2014, doi:10.1016/j.jbo.2013.12.001
- Internet – Web Pathology portal. Created by: Dharam Rammani, MD https://www.webpathology.com/case.asp?case=663
Who is at risk?
The following are at risk of developing hyperparathyroidism:
- persons with long-term and severe vitamin D or calcium deficiency;
- women during their menopause;
- persons taking lithium preparations;
- persons suffering from rare hereditary diseases in which the activity of part of the endocrine glands is disrupted;
- patients undergoing radiation therapy.
The following are at risk of developing hypoparathyroidism:
- persons whose close relatives suffer from this disease;
- persons who have undergone certain surgical interventions in the neck area, especially removal of the thyroid gland;
- persons suffering from autoimmune diseases (with such diseases, cells of the immune system begin to destroy the structures of their own body);
- patients undergoing radiation therapy.
Forecast
In the absence of treatment and severe course of the disease, disability occurs. Life-threatening is hypercalcemic crisis , the mortality rate of which reaches 60%. Detection of the disease at an early stage and timely surgical treatment prevent the occurrence of osteovisceral complications.
After surgery, the symptoms of the disease disappear, recovery occurs in 90% of cases. During the first year after surgery, bone mineral density increases by 15-25%.