Compound
One Ambrohexal tablet contains ambroxol hydrochloride (active ingredient) - 0.03 g + excipients ( calcium hydrogen phosphate, corn starch, magnesium stearate, lactose monohydrate, sodium carboxymethyl starch, colloidal silicon dioxide ).
Capsules (extended release) contain active ingredient - 0.075 g + excipients ( eudragide RL30D, triethyl citrate, titanium dioxide, eudrahydride RS30D, red iron oxide, MCC, magnesium stearate ).
Ambrohexal syrup. For 5 ml of syrup, the active substance is 0.015 mg + excipients ( benzoic acid, raspberry essence, citric acid monohydrate, sorbitol solution, polyvidone, sodium cyclamate, sodium metabesulfite, water, sodium hydroxide, glycerol ).
A solution for inhalation and oral administration (one milliliter) contains the active substance - 7.5 mg + excipients ( citric acid, methyl parahydroxybenzoate, sodium disulfite, sodium hydroxide, purified water ).
Ambrohexal solution for oral administration and inhalation 7.5 mg/ml 50 ml No. 1
Name
Ambrohexal solution d/ing 50ml
Main active ingredient
Ambroxol
Release form
Solution
Compound
1 ml of solution (about 20 drops) contains: active substance – ambroxol hydrochloride 7.5 mg; excipients: methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), sodium metabisulfite (E223), citric acid anhydride, sodium hydroxide, purified water.
Description
A clear, colorless solution, free from undissolved foreign particles upon visual inspection.
Dosage
50ml
Pharmacological properties
Pharmacodynamics
It has a secretomotor (improving sputum transport) and secretolytic effect (reducing the viscosity of sputum), facilitates expectoration; stimulates the serous cells of the glands of the bronchial mucosa, increases the content of mucous secretion and the release of surfactant in the alveoli and bronchi; normalizes the disturbed ratio of serous and mucous components of sputum. By activating hydrolyzing enzymes and enhancing the release of lysosomes from Clark cells, it reduces the viscosity of sputum. Increases the motor activity of the cilia of the ciliated epithelium, increases the mucociliary transport of sputum. After oral administration, the effect occurs within 30 minutes and lasts for 6-12 hours.
Pharmacokinetics
Absorption Over the therapeutic dose range, absorption of ambroxol hydrochloride from immediate-release oral dosage forms is rapid and complete and linear. The time to reach maximum concentration is 1-2.5 hours after oral administration. Distribution The distribution of ambroxol hydrochloride from the blood into the tissue occurs quickly, with the maximum concentration of the active substance found in the lungs. The apparent volume of distribution after oral administration is 552 liters. Plasma protein binding is about 90%. Metabolism About 30% of the prescribed dose is eliminated during the first pass as a result of first-pass metabolism. Ambroxol hydrochloride is metabolized in the liver by glucuronidation and breakdown to dibromanthranilic acid (approximately 10% of the dose). Studies of human liver microsomes have shown that CYP3A4 is responsible for the metabolism of ambroxol hydrochloride to dibromanthranilic acid. Excretion 3 days after administration, approximately 6% of ambroxol hydrochloride is excreted unchanged and approximately 26% is excreted by the kidneys in the form of its conjugates. The terminal half-life of ambroxol hydrochloride is approximately 10 hours. The total clearance is 660 ml/min, with renal clearance accounting for approximately 8% of the total clearance. It was estimated that after 5 days, 83% of the total (radiolabeled) dose was excreted in the urine. Special groups of patients In patients with liver failure, the excretion of ambroxol hydrochloride is reduced. As a result, plasma levels increase by 1.3-2 times. Due to the high therapeutic index of the drug, no dose adjustment is required. The age and gender of patients do not have a clinically significant effect on the pharmacokinetics of ambroxol hydrochloride. Therefore, separate dosing recommendations are not required. Food does not affect the bioavailability of ambroxol hydrochloride. Preclinical safety data Ambroxol has a low level of acute toxicity. Specific oral administration: No toxic effects were observed upon repeated dosing in rats (52 and 78 weeks), rabbits (26 weeks), mice (4 weeks), and dogs (52 weeks). No Observed Adverse Effect Level (NOAEL) in in rats was 50 mg/kg/day, in rabbits - 40 mg/kg/day, in mice - 150 mg/kg/day and 10 mg/kg/day in dogs. Intravenous use: toxicity studies of ambroxol hydrochloride over 4 weeks in rats (4, 16 and 64 mg/kg
infusion 3 hours/day
) and in dogs (45, 90 and 120 mg/kg/day
infusion 3 hours/day
) showed no significant local or systemic toxicity, including histopathology. All adverse events were reversible. Ambroxol hydrochloride was not embryotoxic or teratogenic at oral doses up to 3000 mg/kg/day in rats and up to 200 mg/kg/day in rabbits. Fertility of male and female rats was not affected at doses up to 1500 mg/kg/day. The NOAEL level in the peri- and postnatal development study was 50 mg/kg/day. Ambroxol hydrochloride was of low toxicity to mothers and kittens at 500 mg/kg/day (delayed body weight development and reduced litter size). In vitro (chromosomal aberration test) and in vivo (micronucleus test in mice) genotoxicity studies did not reveal any mutagenic potential of ambroxol hydrochloride. Ambroxol hydrochloride was not tumorigenic in carcinogenicity studies in mice (50, 200, and 800 mg/kg/day) and rats (65, 250, and 1000 mg/kg/day) as a dietary supplement for 105 and 116 weeks, respectively.
Indications for use
Acute and chronic diseases of the respiratory tract with the release of viscous sputum: acute and chronic bronchitis, pneumonia, chronic obstructive pulmonary disease, bronchial asthma with difficulty in sputum discharge, bronchiectasis.
Contraindications
- hypersensitivity to ambroxol hydrochloride or excipients of the drug; - children under 6 years of age (allowed only as prescribed by the attending physician); - pregnancy (first trimester).
Use during pregnancy and lactation
Pregnancy Ambroxol hydrochloride passes through the placental barrier. Preclinical studies have not revealed direct or indirect adverse effects on pregnancy, embryo/fetal development, childbirth or postnatal development. Extensive clinical experience with the use of the drug after the 28th week of pregnancy does not indicate any harmful effects on the fetus. However, normal precautions should be taken regarding the use of medications during pregnancy. In the first trimester of pregnancy, the use of the drug is contraindicated. In the II-III trimesters of pregnancy, it is permissible to use the drug with caution, and the potential benefit to the mother and the possible risk to the fetus should be assessed. Breastfeeding Animal studies have shown that ambroxol hydrochloride passes into breast milk and is therefore not recommended during breastfeeding. Fertility Preclinical studies do not indicate any direct or indirect harmful effects on fertility.
Directions for use and doses
Oral administration (1 ml = 20 drops). Children over 12 years of age and adults: the first 2-3 days - 3 times 4 ml (30 mg ambroxol hydrochloride) per day, then - 2 times 4 ml. If necessary, to increase effectiveness, the dose for adults and adolescents over 12 years of age can be increased by using 8 ml of solution 2 times a day (corresponding to 120 mg of ambroxol hydrochloride / day). Children aged 6-12 years: 2-3 times a day, 2 ml (15 mg ambroxol hydrochloride). Children aged 2-5 years: 3 times a day, 1 ml (7.5 mg of ambroxol hydrochloride) AmbroHEXAL® should be taken after meals diluted with tea, fruit juices, milk or water. During treatment, it is necessary to drink plenty of fluids (juices, tea, water) to enhance the mucolytic effect of the drug. Use for inhalation: Adults and children over 6 years of age are recommended to inhale 1-2 times a day, 2-3 ml (40-60 drops, which corresponds to 15-22.5 mg of ambroxol hydrochloride); For inhalation, you must use a suitable device (for example, a compression or ultrasonic nebulizer). The steam inhaler is not suitable for the use of AmbroHEXAL®. You must follow the instructions for using the device provided by its manufacturer. Heating the solution to 80 °C for 10 minutes does not affect stability. AmbroHEXAL® is mixed with saline and beta-sympathomimetics. AmbroHEXAL® should not be mixed with cromoglicic acid. In addition, mixing should not be carried out with other solutions if the result is a mixture with a pH higher than 6.3, for example, with alkaline saline inhalation solution. An increase in pH may result in precipitation of ambroxol free base or clouding of the solution. To achieve optimal humidification of inhaled air, especially when using a respirator, AmbroHEXAL® 7.5 mg/ml solution for oral administration and inhalation should be mixed with saline in a 1:1 ratio. Ambroxol solution is isotonic and therefore well compatible with mucous membranes. However, coughing may occur if aerosols are inhaled too deeply. Therefore, when inhaling, you should inhale and exhale normally. Before use, the inhalation solution should be warmed to body temperature. The duration of therapy depends on the severity of the disease and is determined by the attending physician. The course of treatment without consulting a doctor is 4-5 days. If the patient has forgotten to take the prescribed dose of the drug, he should postpone taking the drug until the next time, in accordance with the dosage regimen. Do not double the dose to make up for a missed dose.
Side effect
Like all medicines, AmbroHEXAL® can cause side effects, although not everyone gets them. The assessment of side effects is based on the frequency of their occurrence: very common: more than 1 in 10 patients common: 1-10 patients in 100 uncommon: 1-10 patients in 1,000 rare: 1-10 patients in 10,000 very rare : less than 1 in 10,000 patients, frequency unknown: cannot be determined from available data. Immune system disorders Rare: hypersensitivity reactions. Not known: anaphylactic reactions, including anaphylactic shock, angioedema and pruritus. Digestive system disorders Common: nausea, oral hypoesthesia. Uncommon: vomiting, diarrhea, dyspepsia and abdominal pain, dry mouth. Very rare: constipation, drooling. Frequency unknown: dry throat. Skin and subcutaneous tissue disorders Rare: rash, urticaria. Not known: severe cutaneous adverse reactions (including erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis and acute generalized exanthematous pustulosis). Nervous system disorders Common: dysgeusia (change in taste). Respiratory, thoracic and mediastinal disorders Common: pharyngeal hypoesthesia. Very rare: rhinorrhea, dry airways, shortness of breath and bronchospasm (usually in patients with known airway hypersensitivity). Frequency unknown: shortness of breath (as a symptom of a hypersensitivity reaction). Renal and urinary tract disorders Very rare: dysuria. General disorders and reactions at the injection site Rare: drug fever. Reporting of Adverse Reactions It is important to report suspected adverse reactions after registration of a medicinal product to ensure continuous monitoring of the benefit-risk profile of the medicinal product. Health care professionals are encouraged to report any suspected adverse drug reactions through national adverse reaction and drug failure reporting systems. If the patient experiences any adverse reactions, he should consult a doctor. This recommendation applies to any possible adverse reactions, including those not listed in the package insert. By reporting adverse reactions, you can help provide more information about the safety of the drug.
Overdose
Symptoms of overdose in humans have not been described. Specific symptoms in cases of accidental overdose and/or medical errors included symptoms similar to adverse reactions and required symptomatic therapy.
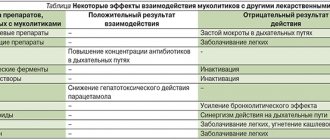
Interaction with other drugs
The use of ambroxol hydrochloride with antitussive drugs leads to impaired sputum discharge while reducing cough, so this combination should not be used. The use of ambroxol hydrochloride with antibiotics (amoxicillin, cefuroxime, erythromycin and doxycycline) increases the concentration of antibiotics in sputum and bronchial secretions. The clinical significance of this has not been established.
Precautionary measures
Severe skin reactions, such as erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis and acute generalized exanthematous pustulosis, have been reported in association with ambroxol hydrochloride. If symptoms or signs of a progressive skin rash appear (sometimes accompanied by the formation of blisters or changes in the mucous membranes), stop taking AmbroHEXAL® immediately and consult a doctor. In most cases, such reactions were associated with the severity of the patient's underlying disease and (or) the use of concomitant medications. Additionally, in the early stages of Stevens-Johnson syndrome or toxic epidermal necrolysis, patients may initially present with flu-like symptoms, such as fever, body aches, runny nose, cough, and sore throat. It is possible that due to confusion by these nonspecific flu-like symptoms, symptomatic treatment was started with cold and cough medications. If you have impaired renal function or severe liver disease, AmbroHEXAL® should be taken only after consulting a doctor. As with any drug with hepatic metabolism followed by renal excretion, accumulation of ambroxol metabolites should be expected in severe renal failure. In patients with impaired bronchial motility and copious bronchial secretions (as, for example, in the rare syndrome of primary ciliary dyskinesia), AmbroHEXAL® should be used with caution due to the risk of difficulty with the discharge of large amounts of sputum and blockage of the bronchi. Since the risk of bronchospastic reactions is mainly due to inhalation, AmbroHEXAL® 7.5 mg/ml, solution for oral administration and inhalation should not be used in patients with a known history of bronchial hyperactivity and/or atopy. The drug contains methyl parahydroxybenzoate, propyl parahydroxybenzoate, which can cause allergic reactions (including delayed ones), in exceptional cases bronchospasm. Sodium metabisulfite, which is part of the drug, in rare cases can cause serious hypersensitivity reactions and bronchospasm.
Storage conditions
Keep out of the reach of children! Store in a place protected from light at a temperature not exceeding 25 °C.
Release form
- Round and flat tablets (white, with rounded edges) with a score line on one side. Packages of 20, 30, 50 and 100 pieces.
- White gelatin capsules with white or pinkish powder inside. Packages of 10, 20, 50 or 100 pieces.
- Transparent, slightly yellowish viscous syrup. Available in 100 or 250 ml bottles, including a measuring spoon.
- Ambrohexal solution for inhalation and oral administration is transparent, colorless. In bottles with a dispenser and a measuring cup of 50 and 100 mg.
Instructions for use AMBROHEXAL® (AMBROHEXAL)
Pills
Adults and children over 12 years of age
in the first 2-3 days of therapy, 30 mg (1 tablet) 3 times a day should be prescribed, then 30 mg 2 times a day.
Children aged 6 to 12 years
Prescribe 15 mg (1/2 tablet) 2-3 times a day.
Extended release capsules
Adults and children over 12 years of age
Prescribe 75 mg (1 capsule) 1 time/day in the morning or evening after meals, without chewing.
Syrup
Adults and children over 12 years of age
should be prescribed in the first 2-3 days, 2 measuring spoons (30 mg) 2-3 times a day, then 2 measuring spoons (30 mg) 2 times a day.
If necessary, to increase effectiveness, adults
can be prescribed 4 scoops (60 mg) up to 2 times a day to achieve a therapeutic effect.
Children aged 6 to 12 years
should be prescribed 1 scoop (15 mg) 2-3 times/day.
Children aged 2 to 6 years
should be prescribed 1/2 measuring spoon (7.5 mg) 3 times a day.
Children under 2 years of age
should be prescribed 1/2 measuring spoon (7.5 mg) 2 times a day.
Oral solution
Adults and children over 12 years old
prescribed 4 ml (80 drops) 3 times/day for the first 2-3 days, then 4 ml (80 drops) 2 times/day.
Children aged 6 to 12 years
It is recommended to prescribe 2 ml (40 drops) 2-3 times/day (30-45 mg/day).
Children aged 2 to 6 years
It is recommended to prescribe 1 ml (20 drops) 3 times/day (22.5 mg/day).
Children under 2 years of age
It is recommended to prescribe 1 ml (20 drops) 2 times/day (15 mg/day).
Inhalations
The oral solution can also be used as inhalation.
Adults and children over 5 years of age
It is recommended to carry out inhalations 1-2 times/day, 40-60 drops (15-45 mg/day).
Children under 5 years of age
It is recommended to inhale 1-2 times/day, 40 drops (15-30 mg/day). For inhalation, an appropriate device should be used.
Patients with impaired renal function or severe liver impairment
it is necessary to increase the interval between doses of Ambrohexal or reduce the dose.
Ambrohexal® should be taken after meals with sufficient liquid.
During treatment, it is necessary to drink plenty of fluids (juices, tea, water) to enhance the mucolytic effect of Ambrohexal.
The duration of treatment is determined by the doctor individually and depends on the severity of the disease. If it is necessary to use the drug for more than 4-5 days, medical supervision is required. Failure to comply with the dosage regimen may reduce the effectiveness of treatment.
Pharmacodynamics and pharmacokinetics
The active ingredient, ambroxol, belongs to the group of benzylamines . Restores the normal balance between the serous and mucous components of the bronchial secretion , by increasing the secretion of the mucous membrane and directly affects the active cells in the bronchial glands. The substance stimulates the activity of special villi of the respiratory tract , thereby facilitating its movement through the bronchi. Ambroxol hydrochloride also has the property of reducing the viscosity of sputum by depolymerizing mucopolysaccharides . The amount of bronchial secretion does not increase, stimulation of centers causing increased cough does not occur.
In a period of time from half an hour to three hours, when taking tablets orally, the drug reaches its maximum concentration in the lungs. About 90% of the substance binds to blood proteins . Most of the drug is excreted by the kidneys 6-11 hours after administration as a glucuronide (or unchanged), the rest is metabolized in the liver tissue.
pharmachologic effect
Mucolytic drug with expectorant action. It has secretomotor, secretolytic and expectorant effects. Stimulates serous cells of the bronchial mucosa, increases the content of mucous secretions and the release of surfactant (surfactant) in the alveoli and bronchi, normalizes the disturbed ratio of serous and mucous components of sputum.
By activating hydrolyzing enzymes and enhancing the release of lysosomes from Clara cells, ambroxol reduces the viscosity of sputum. Increases the motor activity of the ciliated epithelium, increases mucociliary transport, and facilitates the removal of mucus from the respiratory tract.
When ambroxol is taken orally, the effect, on average, occurs after 30 minutes and lasts 6-12 hours, depending on the size of the single dose.
Side effects
- nausea, epigastric pain , dry mouth , diarrhea ;
- dryness in the nasal cavity or, on the contrary, excessive secretion;
- allergic reactions, incl. on the skin;
- general weakness , dysuria .
metabisulfite may develop ( diarrhea, shock, loss of consciousness, nausea, asthmatic attacks ).
Instructions for use of Ambrohexal (Method and dosage)
When taking tablets, the daily dose for an adult (over 12 years of age) in the first three days is 90 mg per day, divided into 3 doses. Further, the dosage can be reduced to 60 mg. Children (from 6 years old) are prescribed 30-45 mg per day.
For extended-release capsules, the main condition is to maintain the integrity of the shell when taken. As a rule, 75 mg of the drug (1 tablet) is prescribed after meals, once a day. Not recommended for use by children under 12 years of age.
The syrup is prescribed for adults (from 12 years old) 30 mg (2 scoops) 3 times a day for the first few days. Next - the same amount 2 times a day. The maximum daily dose is 60 mg.
Children from 5 years old - 1 spoon 3 times a day, from 2 to 5 - half a spoon, up to 2 years old - half a spoon 2 times a day.
According to the instructions for Ambrohexal for inhalation , adults are prescribed 80 drops 3 times a day in the first days, then 80 drops 2 times a day. Children under 12 years old - 40 drops 3 times a day, up to 5 years old - 20 drops 3 times a day, up to 2 years old - 20 drops 2 times a day.
The solution for oral administration and inhalation can be diluted in tea, juice, milk or water.
How to dilute drops for inhalation?
The medicine should be diluted with 0.9% saline. solution , in equal proportions approximately 50% to 50%. The prepared solution should be heated to 40-50 degrees. Children should inhale the vapor for 2-3 minutes, adults – up to five minutes.
For adults (over 5 years old), inhalations are made twice a day, diluted with 50 drops.
For children under 5 years old, dilute 40 drops, inhalation – 2 times a day.
You should not use the drug in any form for more than five days, without supervision or consultation with a doctor.
Directions for use and doses
During treatment with Ambrohexal®, it is necessary to drink plenty of fluids (juices, tea, water) to enhance the mucolytic effect of the drug.
The duration of treatment with Ambrohexal is determined by the doctor individually and depends on the severity of the disease. If it is necessary to use the drug for more than 4-5 days, consult a doctor.
- Ingestion
The drug should be taken orally after meals, diluted with tea, fruit juices, milk or water.
1 ml of solution (20 drops) contains 7.5 mg of ambroxol hydrochloride.
Adults and children over 12 years of age are prescribed 4 ml (80 drops) 3 times/day (90 mg/day) in the first 2-3 days, then 4 ml (80 drops) 2 times/day (60 mg/day ).
Children aged 5 to 12 years are prescribed 2 ml (40 drops) 2-3 times a day (30-45 mg/day).
Children aged 2 to 5 years are prescribed 1 ml (20 drops) 3 times a day (22.5 mg/day).
Children under 2 years of age are prescribed 1 ml (20 drops) 2 times a day (15 mg/day). The drug is prescribed only under the supervision of a doctor.
- Use in the form of inhalations
Adults and children over 5 years of age are recommended to inhale 1-2 times a day, 2-3 ml (40-60 drops, which corresponds to 15-45 mg of ambroxol).
Children under 5 years of age are recommended to inhale 2 ml (40 drops, which corresponds to 15-30 mg of ambroxol) 1-2 times a day.
The inhalation solution can be used using any modern inhalation equipment (except steam inhalers). The drug is mixed with saline; to achieve an optimal level of air humidification in the respirator, the drug can be diluted in a 1:1 ratio. Since during inhalation therapy, a deep breath can provoke coughing impulses, inhalations should be carried out in normal breathing mode. Before inhalation, it is usually recommended to warm the inhalation solution to body temperature. For patients with bronchial asthma, inhalation may be recommended after taking bronchodilators.
Interaction
Can be combined with drugs prescribed for bronchial asthma .
antibiotics ( erythromycin, amoxicillin, cefuroxime with caution ; this will increase their concentration in the body.
It is not recommended to combine with codeine and other antitussives .
special instructions
Ambroxol should not be taken simultaneously with antitussive drugs that can inhibit the cough reflex, for example, codeine, because this may make it difficult to remove liquefied mucus from the bronchi.
Ambroxol should be used with caution in patients with a weakened cough reflex or impaired mucociliary transport due to the possibility of sputum accumulation.
Patients taking ambroxol are not recommended to perform breathing exercises due to difficulty in sputum discharge. In critically ill patients, aspiration of liquefied sputum should be performed.
You should not take ambroxol immediately before bed.
In patients with bronchial asthma, ambroxol may increase cough.
Analogs
Level 4 ATX code matches:
Mukolik
Abrol
Ambrosan
Bronchorus
ACC 100
ACC 200
ACC Long
ACC
Mukolwan
Lazolvan
Bromhexine 8
Bromhexine 8 Berlin-Chemie
Bromhexine
Bronchobos
Carbocisteine
Erdomed
Pulmozyme
Pectolvan C
Halixol
Ambrobene
Abrol, ambroxol, ambronorm, bronchoval, flavamed for cough, ambrosal, lasolvan, lazongin, ambrobene, ambrolitin, ambrospray, ambrochem, bronchoval, lazolex, mucosol, medox, pulmibrom, mucotablin, medovent.
Lazolvan or Ambrohexal - which is better?
Both drugs contain the same active ingredient ambroxol. The effectiveness of the impact is approximately the same. The cost of Ambrohexal is lower than that of its analog Lazolvan .
Reviews about Ambrohexal
Reviews for Ambrohexal tablets are good. Helps against severe dry cough, has virtually no side effects. Many consider the advantage of the drug to be its low cost and high availability. The syrup has approximately the same popularity and characteristics, and has a pleasant sweet taste. As a rule, the cough goes away within a week after starting treatment with the drug.
Reviews of inhalation with the solution are positive. Convenient release form, high efficiency and speed of recovery are the main advantages of the drug. Many people use inhalations with Ambrohexal and saline solution for young children and are happy with the results. The disadvantage is the unpleasant taste of this form of the drug.
Price (where to buy)
The cost of Ambrohexal tablets is about 84 rubles for 20 pieces.
Long-acting capsules will cost 120 rubles for 10 pieces.
A bottle of liquid for inhalation costs about 92 rubles per 50 ml.
The syrup costs approximately 103 rubles per 100 ml.
- Online pharmacies in RussiaRussia
ZdravCity
- Ambrohexal tablets 30 mg 20 pcs. Salutas Pharma GmbH
RUB 109 order