Home | About us | Delivery | Advertisers | Login | Registration
The pharmacy is closed on Sundays and holidays.
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2021 Pharmacy 84.
> Home > Drugs > Full list > Over-the-counter drugs| Flavamed® Max | Flavamed® Forte |
Trade proprietary name: Flavamed® International nonproprietary name: ambroxol Chemical name: trans-4-[(2-amino-3,5-dibromobenzyl)amino]cyclohexanol hydrochloride Dosage form: oral solution
Composition: 5 ml of solution contains: Active ingredient: ambroxol hydrochloride – 15.00 mg; Excipients: benzoic acid – 5.75 mg, glycerol 85% – 500.00 mg, sorbitol 70% (non-crystallizing) – 2500.00 mg, hyaetellose (hydroxyethylcellulose) (degree of molar substitution 2.5; average viscosity 6000 mPa c) – 5.00 mg, raspberry flavor No. 516028 – 5.00 mg, purified water – 2719.25 mg.
Description: clear, colorless or brownish liquid with a raspberry odor.
Pharmacotherapeutic group: Mucolytic expectorant.
ATX code: R05CB06.
Pharmacological properties Pharmacodynamics Ambroxol is an active N-demethylated metabolite of bromhexine. It has secretomotor, secretolytic and expectorant effects. Stimulates the serous cells of the glands of the bronchial mucosa, increasing the content of mucous secretions and, thus, normalizing the disturbed ratio of serous and mucous components of sputum. Ambroxol increases the content of mucous secretions, the formation of surfactant (surfactant) and its content in the alveoli and bronchi. Increases the motor activity of the ciliated epithelium, increases mucociliary transport of sputum. On average, when taken orally, the effect occurs within 30 minutes, the duration of action is 6-12 hours, depending on the single dose. Pharmacokinetics Absorption. After oral administration, ambroxol is quickly and almost completely absorbed from the gastrointestinal tract. The maximum concentration in blood plasma (Cmax) is reached after approximately 1-3 hours. The absolute bioavailability of ambroxol when taken orally as a result of metabolism associated with the first-pass effect through the liver is reduced by approximately 1/3. The resulting metabolites (such as dibromoantranilic acid, glucuronides) are excreted by the kidneys. Distribution. Binding to plasma proteins is approximately 80 - 90%. Ambroxol penetrates the blood-brain and placental barriers and is also excreted in breast milk. Metabolism. Ambroxol is metabolized in the liver by conjugation to form pharmacologically inactive metabolites. Excretion. The half-life from blood plasma (T1/2) is 7-12 hours. T1/2 of ambroxol and its metabolites (in total) is about 22 hours. About 90% is excreted by the kidneys, mainly in the form of metabolites. Less than 10% of the amount excreted through the kidneys is ambroxol in unchanged form. In case of severe renal dysfunction (creatinine clearance less than 30 ml/min), accumulation of ambroxol metabolites is possible; in severe liver failure, the clearance of ambroxol is reduced by 20-40%. Due to the high degree of binding to plasma proteins and the large volume of distribution, as well as the slow reverse distribution from tissues to the blood, significant elimination of ambroxol by dialysis or forced diuresis should not be expected.
Indications for use Impaired secretion and transport of sputum in acute and chronic respiratory diseases: - acute and chronic bronchitis; - pneumonia; — chronic obstructive pulmonary disease; - bronchial asthma; - bronchiectasis.
Contraindications - hypersensitivity to ambroxol and other components of the drug (see section "Composition"); - hereditary fructose intolerance; — First trimester of pregnancy and breastfeeding period.
With caution - impaired bronchial motility and increased mucus secretion (for example, in the rare syndrome of primary ciliary dyskinesia); - severe renal failure (creatinine clearance less than 30 ml/min) and/or severe liver failure; - peptic ulcer of the stomach and duodenum, including a history of it; — II and III trimesters of pregnancy; - children under 2 years of age (used only as prescribed by a doctor).
Use during pregnancy and breastfeeding Use of the drug Flavamed®
Contraindicated in the first trimester of pregnancy and during breastfeeding. The use of the drug in the second and third trimesters of pregnancy is possible only after a careful assessment of the balance between the benefits of treatment and possible risks.
Directions for use and dosage Oral solution. Unless otherwise prescribed, the following dosages are recommended: Children under 2 years of age: 1⁄2 scoop (2.5 ml) of Flavamed® solution 2 times daily (equivalent to 15 mg ambroxol hydrochloride/day). Children aged 2 to 5 years: 1⁄2 scoop (2.5 ml) of Flavamed® solution 3 times a day (corresponding to 22.5 mg of ambroxol hydrochloride/day). Children aged 5 to 12 years: 1 scoop of Flavamed® solution
2 - 3 times a day (which corresponds to 30 - 45 mg of ambroxol hydrochloride / day). Adults and children over 12 years of age: during the first 2-3 days, 2 scoops (10 ml) of Flavamed® solution 3 times a day (which corresponds to 90 mg of ambroxol hydrochloride/day), then 2 scoops (10 ml) Flavamed® solution 2 times a day (which corresponds to 60 mg of ambroxol hydrochloride/day). If necessary, to enhance the therapeutic effect, adults can take 4 scoops (20 ml) of Flavamed® solution 2 times a day (which corresponds to 120 mg of ambroxol hydrochloride/day). In case of severe renal and liver failure, the duration of the interval between doses should be increased or the dose of Flavamed® should be reduced.
Flavamed® solution is taken after meals; use a measuring spoon to dose the drug. The duration of use is determined individually depending on the indications and course of the disease. Without a doctor's prescription, Flavamed® should not be taken for more than 4-5 days.
Side effects Possible side effects are listed below in descending frequency of occurrence: very common (> 1/10), common (> 1/100, <1/10), uncommon (> 1/1000, <1/100), rare (> 1/10000, <1/1000), very rare (<1/10000), including isolated reports. Gastrointestinal disorders Common: nausea; Uncommon: vomiting, dry mouth, diarrhea, dyspepsia, abdominal pain. Immune system disorders Uncommon: fever; Rarely: skin rash, urticaria; In isolated cases: anaphylactic reactions up to the development of shock, angioedema, skin itching and other hypersensitivity reactions. Skin and subcutaneous tissue disorders Very rare: epidermal necrolysis; Steven-Johnson syndrome (see section "Special instructions"). Nervous system disorders Common: dysgeusia (impaired sense of taste). Disorders of the respiratory system, chest and mediastinal organs Often: decreased sensitivity in the oral cavity and pharynx; In isolated cases: dryness of the pharyngeal mucosa.
Overdose Symptoms: Specific symptoms of ambroxol overdose in humans have not been described. The observed symptoms of overdose were consistent with the known side effects of ambroxol used in recommended doses (nausea, vomiting, abdominal pain, diarrhea, dyspepsia). Treatment: artificial vomiting, gastric lavage in the first 1-2 hours after taking the drug; intake of fat-containing foods; symptomatic therapy. Due to the high degree of binding of ambroxol to plasma proteins (80-90%), forced diuresis and hemodialysis are ineffective.
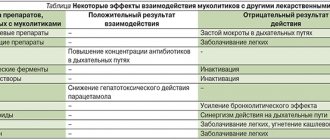
Interaction with other drugs When using ambroxol and antitussive drugs, for example, codeine, due to the suppression of the cough reflex, there may be a risk of accumulation of sputum in the respiratory tract with difficulty in removing it, therefore the simultaneous use of ambroxol and antitussive drugs should be carried out with extreme caution. Ambroxol increases the penetration into the bronchial lumen of amoxicillin, cefuroxime, erythromycin and doxycycline.
Special instructions There is evidence of the occurrence in very rare cases of Stevens-Johnson syndrome and toxic epidermal necrolysis when using ambroxol. If allergic reactions occur, you should immediately stop using the drug and consult a doctor. In severe renal failure (creatinine clearance less than 30 ml/min), the risk of accumulation of ambroxol metabolites must be taken into account. Mucolytics can damage the mucous barrier of the gastrointestinal tract, so ambroxol should be used with caution in patients with gastric and duodenal ulcers, including those with a history. To maintain the secretolytic effect during the period of use of the drug Flavamed®, it is necessary to ensure that a sufficient amount of fluid enters the body. The drug Flavamed® contains sorbitol (sorbitol), therefore its use in patients with hereditary fructose intolerance is contraindicated. Information for patients with diabetes: 5 ml of solution (1 scoop) contains 1.75 g of sorbitol, which corresponds to 0.15 bread units (XU). Effect on the ability to drive vehicles and operate machinery The drug does not affect the performance of potentially hazardous activities that require increased attention and speed of psychomotor reactions.
Release form Oral solution 15 mg/5 ml. 60, 100 or 200 ml of solution in dark glass bottles (type III), sealed with a screw cap. 1 bottle complete with a measuring spoon along with instructions for use in a cardboard box.
Storage conditions At a temperature not higher than 25 oC. Keep the medicine out of the reach of children.
Shelf life 3 years in original packaging. 6 months - after the first opening of the bottle. Do not use after the expiration date stated on the packaging.
Conditions for dispensing from pharmacies Without a prescription.
Manufacturer Berlin-Chemie AG Glienicker Weg, 125 12489 Berlin, Germany
Berlin-Chemie AG Glieniker Weg 125 12489 Berlin, Germany
Address for filing claims: 123317, Moscow, Presnenskaya embankment, building 10, BC “Tower on Naberezhnaya”, block B, tel., fax.
Pharmacokinetics
Suction
Ambroxol after oral administration is quickly and almost completely absorbed. Tmax in blood plasma ranges from 1 to 3 hours. The absolute bioavailability of ambroxol when taken orally as a result of metabolism associated with the “first pass” effect through the liver decreases by approximately 1/3.
Distribution
Plasma protein binding is 80-90%.
Ambroxol passes through the placental barrier, the BBB, and is excreted in breast milk.
Removal
The final T1/2 is 7-12 hours. T1/2 from plasma of ambroxol and its metabolites (in total) is approximately 22 hours.
Excretion occurs 90% through the kidneys in the form of metabolites formed in the liver (dibromanthranilic acid, glucuronides). Less than 10% of the amount excreted through the kidneys is ambroxol in unchanged form.
Pharmacokinetics in special clinical situations
In severe renal impairment, T1/2 of ambroxol metabolites increases.
special instructions
In case of impaired bronchial motility and a significant amount of secretion (for example, with cilia dysfunction syndrome), Flavamed should be used with caution due to possible stagnation of secretion.
In case of impaired renal or liver function, the drug is prescribed by increasing the intervals between doses or reducing the dose.
In severe renal failure, the possibility of accumulation of ambroxol metabolites formed in the liver should be taken into account.
1 measuring spoon (5 ml of solution) contains 1.75 g of sorbitol, which corresponds to 0.15 XE. The energy value is 2.6 kcal/g sorbitol. Sorbitol may have a mild laxative effect.
Use in pediatrics
Use in children under 2 years of age
in the form of a cough solution for oral administration should be carried out under strict medical supervision.
Impact on the ability to drive vehicles and operate machinery
No data on impact.
pharmachologic effect
Mucolytic and expectorant drug. Ambroxol is an active N-demethylated metabolite of bromhexine. It has secretolytic and secretomotor effects. After taking ambroxol, the proportion of serous bronchial secretion increases. Reduces the viscosity of bronchial secretions and stimulates the activity of the ciliated epithelium, promoting the discharge of mucus.
Ambroxol increases the synthesis and secretion of surfactant, as well as the permeability of the bronchial vascular barrier.
The effect occurs after 30 minutes and lasts 6-12 hours depending on the single dose.
Dosage regimen
Children under 2 years of age
Prescribe 2.5 ml of cough solution for oral administration 2 times a day (corresponding to 15 mg of ambroxol hydrochloride/day).
Children aged 2 to 5 years
Prescribe 2.5 ml of cough solution for oral administration 3 times a day (corresponding to 22.5 mg of ambroxol hydrochloride/day).
Children aged 6 to 12 years
Prescribe 5 ml of cough solution for oral administration or 1/2 tablet. 2-3 times/day (corresponds to 30-45 mg of ambroxol hydrochloride/day).
Adults and children over 12 years of age
Prescribed during the first 2-3 days, 10 ml of cough solution for oral administration or 1 tablet. 3 times/day (corresponds to 90 mg of ambroxol hydrochloride/day), then 10 ml of cough solution for oral administration or 1 tablet. 2 times/day (corresponds to 60 mg ambroxol hydrochloride/day).
If necessary, the dose can be increased to 20 ml of cough solution for oral administration or 2 tablets. 2 times/day (corresponds to 120 mg ambroxol hydrochloride/day).
The secretolytic effect of ambroxol hydrochloride is maintained by fluid intake, so it is necessary to consume a sufficient amount of fluid during treatment.
The drug should be taken after meals. Cough solution for oral administration is dosed with a measuring spoon included in the package. The tablets are taken orally without chewing.
The duration of use is determined individually depending on the indication and course of the disease. Without a doctor's recommendation, Flavamed should not be taken for more than 4-5 days.