Erythromycin (in Latin Erythromycin) is a mixture of structurally similar compounds that are produced by the bacterium Saccharopolyspora erythraea. In medicine, erythromycin is used as an antibiotic. From a chemical point of view, the drug is a representative of the glycoside class; from a pharmacological point of view, it belongs to macrolides. The main component of the drug is erythromycin A, then a little erythromycin B (5%) and an even smaller amount of erythromycin C.
Saccharopolyspora erythraea
Erythromycin is most often used to treat gram-positive (streptococci, staphylococci), anaerobic (Propionibacterium, Corynebacterium) and mycoplasma infections.
Chemical properties[edit | edit code]
Macrolides get their name from the macrocyclic lactone ring (14-membered in erythromycin and clarithromycin and 15-membered in azithromycin), to which at least one deoxysugar residue is connected. Clarithromycin differs from erythromycin in the methyl group that replaces the hydrogen of the hydroxyl group at position 6, and azithromycin has an additional nitrogen atom with a methyl group attached to its lactone ring. Due to these structural differences, azithromycin and clarithromycin are more stable in an acidic environment, penetrate tissue better and have a wider spectrum of action. The chemical formulas of macrolides are as follows:
Structural formula of erythromycin Structural formulas of clarithromycin and azithromycin
Antimicrobial activity[edit | edit code]
Erythromycin usually has a bacteriostatic effect, but in high concentrations it can act bactericidal on highly sensitive microorganisms. In vitro, erythromycin is most active against aerobic gram-positive cocci and rods (Steigbiegel, 2000). The MIC for sensitive strains of Streptococcus pyogenes and Streptococcus pneumoniae ranges from 0.015 to 1 μg/ml. However, the number of erythromycin-resistant streptococcal strains is increasing. The mechanism of resistance is the same for all macrolides, therefore such strains are cross-resistant to other drugs in this group. Due to the widespread use of macrolides, the proportion of Streptococcus pyogenes strains resistant to them can reach 40% (Seppala et al., 1997; Esposito et al., 1998). In Streptococcus pneumoniae, the prevalence of macrolide resistance is particularly high among penicillin-resistant strains, being 60% compared with 5% among penicillin-susceptible strains (Thomsberry et al., 1997; Thomsberry et al., 1999). The MIC of erythromycin for viridans streptococci is 0.06–3.1 μg/ml.
Some staphylococci are sensitive to erythromycin, but the MIC for them varies widely (for Staphylococcus epidermidis - from 8 to more than 32 μg/ml, for Staphylococcus aureus - from 0.12 to more than 128 μg/ml). Hospital strains of Staphylococcus aureus are often resistant to macrolides; in addition, Staphylococcus aureus may become resistant during treatment. Macrolide-resistant strains of Staphylococcus aureus exhibit cross-resistance to clindamycin (Fass, 1993). Many gram-positive bacilli are sensitive to erythromycin: the MIC for Clostridium perfringens is 1 μg/ml, for Corynebacterium diphtheriae - 0.2-3 μg/ml, for Listeria monocytogenes - 0.25-4 μg/ml.
Erythromycin has no effect on most enterobacteria, but is active against other gram-negative microorganisms. In vitro, it is moderately active against Haemophilus influenzae (MIC 1-32 μg/ml) and Neisseria meningitidis (MIC 0.4-1.6 μg/ml), highly active against most strains of Neisseria gonorrhoeae (MIC 0.12-2 μg/ml). ml; Steigbigel, 2000). In addition, it acts on Pasteurella mul-tocida, Borrelia spp. and Bordetella pertussis. Strains of Bacteroides fragilis are often resistant to erythromycin (MIC 2–32 μg/ml), and Campylobacter jejuni are sensitive (MIC 0.5–4 μg/ml). Erythromycin is effective against infections caused by Mycoplasma pneumoniae (MIC 0.004–0.02 μg/ml) and Legionella pneumophila (MIC 0.01–2 μg/ml). For most strains of Chlamydia trachomatis, the MIC is 0.06–2 μg/ml. In vitro, some atypical mycobacteria, including Mycobacterium scrofulaceum, are also sensitive to erythromycin. The susceptibility of Mycobacterium kansasii and Mycobacterium avium-intracellulare varies (Molavi and Weinstein, 1971). Mycobacterium fortuitum is resistant to erythromycin.
Clarithromycin is slightly more active than erythromycin against strains of streptococci and staphylococci that are sensitive to the latter, and is moderately active against Haemophilus influenzae and Neisseria gonorrhoeae. In addition, clarithromycin has a good effect on Moraxella catarrhalis, Chlamydia spp., Legionella pneumophila, Borrelia burgdorferi, Mycoplasma pneumoniae, Mycobacterium leprae (Chan et al., 1994).
Azithromycin is generally less active than erythromycin against gram-positive bacteria (streptococci, enterococci), but slightly stronger than erythromycin and clarithromycin against Haemophilus influenzae and Campylobacter spp. (Peters et al., 1992). Azithromycin is highly active against Moraxella catarrhalis, Pasteurella multocida. Chlamydia spp., Mycoplasma pneumoniae. Legionella pneumophila, Borrelia burgdorferi, Fusobacterium spp. and Neisseria gonorrhoeae.
A microorganism is considered sensitive to new macrolides (clarithromycin and azithromycin) if its MIC does not exceed 2 μg/ml. An exception is Haemophilus influenzae: the MIC for strains sensitive to clarithromycin does not exceed 8 μg/ml, and the MIC for strains sensitive to azithromycin is 4 μg/ml.
Azithromycin and clarithromycin are more active than erythromycin against Mycobacterium avium-intracellulare. New macrolides also act on some protozoa (Toxoplasma gondii, Cryptosporidium spp., Plasmodium spp.).
Erythromycin tablets po enteric soluble 500 mg No. 5x2
Name
Erythromycin tablet, coated, vol. 500 mg in container pack No. 5x2
Description
Tablets, film-coated, enteric-coated, white or almost white, oval, biconvex; A cross section shows one white layer.
Main active ingredient
Erythromycin
Release form
Tablets, film-coated, enteric-coated, white or almost white, oval, biconvex; A cross section shows one white layer.
Dosage
500 mg №5x2
special instructions
Use with caution in case of impaired liver and/or kidney function. Drugs that increase gastric acidity and acidic drinks inactivate erythromycin. Erythromycin should not be taken with milk or dairy products.
pharmachologic effect
Antibiotic of the macrolide group. Has a bacteriostatic effect. However, in high doses it has a bactericidal effect against sensitive microorganisms. Erythromycin reversibly binds to bacterial ribosomes, thereby inhibiting protein synthesis. Active against gram-positive bacteria: Staphylococcus spp. (strains producing and not producing penicillinase), Streptococcus spp. (including Streptococcus pneumoniae); gram-negative bacteria: Neisseria gonorrhoeae, Haemophilus influenzae, Bordetella pertussis, Brucella spp., Legionella spp., Bacillus anthracis, Corynebacterium diphtheriae; anaerobic bacteria: Clostridium spp. Erythromycin is also active against Mycoplasma spp., Chlamydia spp., Spirochaetaceae, Rickettsia spp. Gram-negative bacilli are resistant to erythromycin, incl. Escherichia coli, Pseudomonas aeruginosa, Shigella spp., Salmonella spp.
Pharmacokinetics
Bioavailability is 30-65%. Distributed in most tissues and body fluids. Plasma protein binding is 70-90%. Metabolized in the liver, partially with the formation of inactive metabolites. T1/2 - 1.4-2 hours. Excreted in bile and urine.
Indications for use
Infectious and inflammatory diseases caused by microorganisms sensitive to erythromycin, incl. diphtheria, whooping cough, trachoma, brucellosis, Legionnaires' disease, tonsillitis, scarlet fever, otitis media, sinusitis, cholecystitis, pneumonia, gonorrhea, syphilis. Treatment of infectious and inflammatory diseases caused by pathogens (in particular, staphylococci) resistant to penicillin, tetracyclines, chloramphenicol, streptomycin. For external use: juvenile acne. For local use: infectious and inflammatory eye diseases.
Directions for use and doses
They are set individually depending on the location and severity of the infection and the sensitivity of the pathogen. In adults, a daily dose of 1-4 g is used. Children under 3 months of age - 20-40 mg/kg/day; at the age of 4 months to 18 years - 30-50 mg/kg/day. Frequency of application: 4 times/day. The course of treatment is 5-14 days, after the symptoms disappear, treatment is continued for another 2 days. Take 1 hour before meals or 2-3 hours after meals. Apply the solution for external use to the affected areas of the skin. The ointment is applied to the affected area, and in case of eye diseases, it is placed behind the lower eyelid. The dose, frequency and duration of use are determined individually.
Use during pregnancy and lactation
Erythromycin crosses the placental barrier and is excreted in breast milk. When using erythromycin during pregnancy, the expected benefit to the mother and the potential risk to the fetus should be assessed. If it is necessary to use it during lactation, the issue of stopping breastfeeding should be decided.
Precautionary measures
Use with caution in case of impaired liver and/or kidney function.
Interaction with other drugs
With the simultaneous use of erythromycin with theophylline, aminophylline, caffeine, an increase in their concentration in the blood plasma is observed and thereby increases the risk of developing toxic effects. Erythromycin increases plasma concentrations of cyclosporine and may increase the risk of nephrotoxicity. Drugs that block tubular secretion prolong T1/2 of erythromycin. Incompatible with lincomycin, clindamycin and chloramphenicol (antagonism). Erythromycin reduces the bactericidal effect of beta-lactam antibiotics (penicillins, cephalosporins, carbapenems). When used simultaneously, erythromycin increases theophylline content. When taken simultaneously with drugs that are metabolized in the liver (carbamazepine, valproic acid, hexobarbital, phenytoin, alfentanil, disopyramide, lovastatin, bromocriptine), the concentration of these drugs in plasma may increase (it is an inhibitor of microsomal liver enzymes). IV administration of erythromycin enhances the effect of ethanol (accelerating gastric emptying and reducing the duration of action of alcohol dehydrogenase in the gastric mucosa). Erythromycin reduces the clearance of triazolam and midazolam and may therefore enhance the pharmacological effects of benzodiazepines. When taken simultaneously with terfenadine or astemizole, arrhythmia may develop (ventricular fibrillation and flutter, ventricular tachycardia, even death); with dihydroergotamine or non-hydrogenated ergot alkaloids, vasoconstriction to spasm and dysesthesia is possible. When used simultaneously, it slows down the elimination (increases the effect) of methylprednisolone, felodipine and coumarin anticoagulants. When co-administered with lovastatin, rhabdomyolysis increases. Erythromycin increases the bioavailability of digoxin. Erythromycin reduces the effectiveness of hormonal contraception.
Contraindications
History of jaundice, severe liver dysfunction, hypersensitivity to macrolides.
Compound
In 1 tab. erythromycin 500 mg Excipients: potato starch, polyvinylpyrrolidone (povidone), Kollidon CL-M (crospovidone), polysorbate 80 (Tween 80), calcium stearate, talc. Shell composition: cellulose acetylphthalyl, medical castor oil, titanium dioxide.
Side effect
From the digestive system: nausea, vomiting, epigastric pain, cholestatic jaundice, tenesmus, diarrhea, dysbacteriosis; rarely - pseudomembranous enterocolitis, impaired liver function, increased activity of liver transaminases, pancreatitis. Allergic reactions: skin rash, urticaria, eosinophilia; rarely - anaphylactic shock. Effects caused by chemotherapy: oral candidiasis, vaginal candidiasis. From the senses: reversible ototoxicity - hearing loss and/or tinnitus (when using high doses - more than 4 g / day). From the cardiovascular system: rarely - tachycardia, prolongation of the QT interval on the ECG, atrial fibrillation and/or flutter (in patients with a prolonged QT interval on the ECG). Local reactions: phlebitis at the site of intravenous administration.
Storage conditions
In a place protected from moisture, at a temperature not exceeding 25 °C.
Mechanism of action[edit | edit code]
Macrolides are bacteriostatic antibiotics that inhibit protein synthesis by reversibly binding to the 505 ribosomal subunit (Fig. 47.3; Brisson-Noel et al., 1988). Macrolides act on the same target as chloramphenicol, competitively inhibiting its binding to ribosomes (Fig. 47.2). A change in the 50S ribosomal subunit due to mutation, which disrupts the binding of macrolides to the target, leads to the development of drug resistance. Unlike chloramphenicol, which prevents the formation of a peptide bond, macrolides act at the stage of translocation - the transfer of a newly synthesized peptidyl-tRNA molecule from the aminoacyl site of the ribosome to the peptidyl site.
Gram-positive bacteria accumulate almost 100 times more erythromycin than gram-negative bacteria.
In an alkaline environment, the antimicrobial activity of the drug is much higher, probably because in its non-ionized form, which predominates at high pH, it penetrates bacterial cells much better (Sabath et al., 1968; Vogel et al., 1971).
Acquired resistance to macrolides is due to three main mechanisms:
- active removal of the drug from the cell (in staphylococci the transporter is encoded by the mrsA gene, in Streptococcus pyogenes - by the mefA gene, in Streptococcus pneumoniae - by the mefE gene),
- a decrease in the affinity of ribosomes for the drug, due to their methylation under the influence of the inducible or constitutive enzyme methyltransferase (this enzyme is encoded by the egtA, egtB and egtC genes)
- hydrolysis of macrolides by enterobacterial esterases (Barth61dmy et al., 1984).
The second mechanism, mediated by the egt genes, determines resistance not only to macrolides, but also to lincosamides and streptogramins (MLSB phenotype). All of these drugs act on the same target, the methylation of which leads to the formation of resistance. There is another mechanism of macrolide resistance found in Bacillus subtilis, Campylobacter spp. and gram-positive cocci. It is caused by chromosomal mutations that change the structure of the protein 508-ribosomal subunit.
Pharmacokinetics[edit | edit code]
Suction[edit | edit code]
Erythromycin in the form of a base is absorbed from the gastrointestinal tract to a sufficient extent, but not completely; absorption occurs in the upper parts of the small intestine. In an acidic environment, the drug is destroyed, so it is produced in the form of tablets in an acid-resistant coating that dissolves in the duodenum, or in the form of capsules containing granules coated with such a coating. When eating, the pH of the intestinal contents decreases and the absorption of the drug slows down. After oral administration at a dose of 250 mg, the maximum serum concentration of erythromycin is achieved after 4 hours and is only 0.3-0.5 μg/ml, and after oral administration at a dose of 500 mg - 0.3-1.9 μg/ml. Erythromycin esters - stearate, estolate and ethyl succinate - are more stable in an acidic environment and are better absorbed, especially erythromycin estolate (food intake has almost no effect on its bioavailability). After oral administration of erythromycin estolate, the maximum serum concentration is achieved after 2 hours; at a dose of 250 mg it is approximately 1.5 μg/ml, and at a dose of 500 mg - 4 μg/ml. In this case, the share of inactive ester accounts for 65-80%, that is, the actual concentration of the active drug is approximately the same as when ingesting erythromycin in the form of a base. Another ester, erythromycin ethyl succinate, is also well absorbed. After oral administration at a dose of 500 mg, the maximum serum concentration of erythromycin ethylsuccinate is achieved after 1-2 hours and is 1.5 μg/ml (active drug concentration is 0.5 μg/ml).
Figure 47.3. The influence of macrolides (erythromycin, clarithromycin and azithromycin) on protein synthesis in bacteria.
For intravenous administration, erythromycin is available in the form of lactobionate and glucoheptonate. The serum concentration of the drug when administered intravenously is higher than when administered orally. 1 hour after administration at a dose of 500-1000 mg, it is about 10 mcg/ml.
Clarithromycin is rapidly absorbed after oral administration, but is largely metabolized during the first passage through the liver, so its bioavailability is only 50-55%. The maximum concentration is reached after approximately 2 hours. Regular (non-long-acting) clarithromycin preparations can be taken both with meals and between meals. The long-acting drug is taken with food (1 g 1 time per day) to increase bioavailability. When administered at a dose of 500 mg every 12 hours, the maximum serum concentration at steady state is 2-3 μg/ml and is achieved 2 hours after administration (Fraschini et al., 1993). When taking long-acting tablets (1 g 1 time per day), this concentration is achieved after 2-4 hours.
Azithromycin is rapidly absorbed after oral administration and penetrates into all tissues and biological fluids, with the exception of the CSF. When taking antacids containing aluminum and magnesium hydroxide simultaneously, the maximum serum concentration of the drug decreases, but the bioavailability does not decrease. Azithromycin should not be taken with food. After taking a saturating dose (500 mg), the maximum serum concentration of the drug is about 0.4 μg/ml. If the drug is subsequently taken at a maintenance dose of 250 mg once a day for 4 days, the maximum serum concentration at steady state will be 0.24 mcg/ml. Azithromycin is also available for intravenous administration. By the end of an hour-long infusion at a dose of 500 mg, the serum concentration of the drug is 3-4 mcg/ml.
Distribution[edit | edit code]
Erythromycin easily penetrates into the intercellular fluid and exhibits antibacterial activity in all tissues and biological fluids, with the exception of the brain and CSF. In the secretion of the prostate gland, the concentration of erythromycin reaches approximately 40% of the serum concentration. The concentration of the drug in middle ear discharge (50% serum concentration) may not be sufficient to treat otitis media caused by Haemophilus influenzae. Erythromycin is 70-80% bound to plasma proteins, and erythromycin estolate is 96% bound. Erythromycin crosses the placenta; its serum concentration in the fetus is approximately 5-20% of the serum concentration in the mother. The drug is found in milk in significant quantities (50% of serum concentration).
Clarithromycin is rapidly metabolized on first pass through the liver to form the active metabolite, 14-hydroxyclarithromycin. Both substances are distributed throughout the body, reaching high concentrations inside cells. Concentrations of clarithromycin and its active metabolite in tissues are usually higher than in serum, and concentrations in middle ear discharge exceed serum concentrations by 50%. The degree of binding of clarithromycin to plasma proteins is 40-70% and depends on the serum concentration of the drug.
The pharmacokinetic properties of azithromycin are unique. It is distributed throughout the body and is present in high concentrations in cells (including phagocytes). As a result, the concentration of the drug in tissues and biological fluids is much higher than in serum. In vivo, azithromycin accumulates in fibroblasts, from which it is likely readily transferred to phagocytes (McDonald and Pruul, 1991). The degree of binding of azithromycin to plasma proteins is low and appears to decrease with increasing serum concentration (at low concentrations this figure is 51%).
Elimination[edit | edit code]
In the active form, only 2-5% of ingested erythromycin is excreted in the urine; with intravenous administration this figure increases to 12-15%. The drug accumulates in the liver and is excreted in active form in the bile, where its content at very high serum concentrations can reach 250 mcg/ml. T1/2 is approximately 1.6 hours. According to some data, with anuria, the elimination of erythromycin slows down, but in patients with renal failure, the dose of the drug is usually not reduced. During peritoneal dialysis and hemodialysis, the drug is excreted insignificantly.
The kidneys and liver are involved in the elimination of clarithromycin. In the liver it is metabolized to form several metabolites. The most important of these, 14-hydroxyclarithromycin, has antimicrobial activity. When high doses of clarithromycin are used, its pharmacokinetics become nonlinear, apparently due to saturation of metabolic reactions (Chu et al., 1992). The main metabolic pathways are stereospecific hydroxylation at position 14 and oxidative N-demethylation. In vivo, stereospecific hydroxylation produces R- and S-isomers, with the R-isomer having higher biological activity and being formed in greater quantities. T1/2 of clarithromycin and 14-hydroxyclarithromycin are 3-7 and 5-9 hours, respectively. As the dose increases, T1/2 increases. From 20 to 40% of clarithromycin is excreted unchanged in the urine, depending on the dose and dosage form (tablets or oral suspension). Another 10-15% comes from 14-hydroxyclarithromycin. In renal and hepatic failure, the pharmacokinetics of clarithromycin changes. However, the dose is reduced only in severe chronic renal failure (GFR less than 30 ml/min).
The pharmacokinetics of azithromycin have not been fully studied. The main route of elimination is excretion in bile; part of the drug is converted in the liver into inactive metabolites. Only 12% of azithromycin is excreted unchanged in the urine. Long T1/2 (40-68 hours) is due to the accumulation and binding of azithromycin in tissues.
Erythromycin recipe in Latin
A prescription for erythromycin in Latin is written in the following forms:
- tablets in dosages of 100, 250, 500 mg;
- oral suspension 200 and 400 mg/5 ml;
- ampoules of 500 or 1000 mg,
- ointment 10 and 15 grams, 100,000 units each.
Read the general rules for writing a prescription in Latin here.
In tablet form
We will write out a prescription for erythromycin in the form of tablets (for more details, see the recipe for tablets in Latin) with a dosage of 100 mg and prescribe 1 tablet to be taken orally 4 times a day:
Rp.: Erythromycini 100 mg Dtd N 20 in tab. S. Orally, 1 tablet 4 times a day.
In the form of a suspension
We will write out a prescription for erythromycin in the form of an oral suspension. For more details, see the recipe for the suspension in Latin and, since the suspensions are produced in bottles, read the recipe for the bottles in Latin.
Rp.: Erythromycini 400 mg/5 ml Dtd N 10 S. Orally, 1 bottle 2 times a day. Shake before use!
In ampoules
We will write out a prescription for erythromycin in the form of ampoules. More details - recipe for ampoules in Latin.
Rp.: Erythromycini 500 mg Dtd N 10 in amp. S. Dilute in water for injection. Intramuscularly, 1 ampoule 2 times a day.
Application[edit | edit code]
Depending on the pathogen and severity of the infection, the dose of erythromycin for oral administration in adults is usually 1-2 g/day in several doses (usually the drug is taken every 6 hours). Even at a dose of 8 g/day orally for 3 months, erythromycin is well tolerated. If possible, erythromycin and erythromycin stearate should not be taken immediately before or immediately after meals (this does not apply to erythromycin estolate and erythromycin ethylsuccinate). In children, the daily oral dose is 30-50 mg/kg in 4 divided doses; for severe infections it can be doubled. IM administration is not recommended as injections are painful. IV medications (erythromycin glucoheptonate or erythromycin lactobionate) are used for severe infections such as Legionnaires' disease. The usual dose is 0.5-1 g IV every 6 hours. When treating erythromycin with glucoheptonate at a dose of 1 g IV every 6 hours for 4 weeks, no significant side effects were observed, with the exception of thrombophlebitis at the site of venipuncture.
Clarithromycin is available in the form of tablets, powder for oral suspension and powder for injection. The drug is usually prescribed 2 times a day. For mild and moderate infections in children over 12 years of age and adults, a single dose is 250 mg. If the infection is severe (eg, pneumonia) or caused by a microorganism against which clarithromycin is not very active (eg, Haemophilus influenzae), the single dose is increased to 500 mg. In clinical trials, children under 12 years of age were prescribed 7.5 mg/kg twice daily. Long-acting tablets containing 500 mg of clarithromycin are taken 2 pieces once a day.
Azithromycin is available in the form of tablets, an oral suspension and a powder for the preparation of an injection solution. The drug is given orally 1 hour before meals or 2 hours after meals. When treating community-acquired pneumonia, pharyngitis, infections of the skin and subcutaneous tissue on an outpatient basis, a saturating dose of 500 mg is given on the first day, and a maintenance dose of 250 mg/day from days 2 to 5. For the treatment and prevention of infection caused by Mycobacterium avium-intracellula in patients with AIDS, higher doses are used. For this purpose, azithromycin is prescribed at a dose of 500 mg/day in combination with one or more other drugs. For primary prevention of this infection, azithromycin is taken at a dose of 1200 mg once a week. For uncomplicated nongonococcal urethritis (presumably caused by Chlamydia trachomatis), 1 g of azithromycin is prescribed as a single dose. At a dose of 2 g once, the drug is effective for gonorrhea; it is not widely used for this purpose (Centers for Disease Control and Prevention, 1998).
For children, azithromycin is prescribed as a suspension for oral administration. For acute otitis media and pneumonia, give 10 mg/kg (no more than 500 mg) on the first day, and 5 mg/kg/day (no more than 250 mg/day) in the next 4 days. For angina and pharyngitis, azithromycin is taken at a dose of 12 mg/kg/day (not more than 500 mg/day) for 5 days.
Mycoplasma infections
. Erythromycin (500 mg orally 4 times a day) reduces the duration of fever, and in mycoplasma pneumonia promotes more rapid normalization of the x-ray picture (Rasch and Mogabgab, 1965). In case of intolerance to oral administration, erythromycin is administered intravenously. Other macrolides and tetracyclines are also effective for mycoplasma infections.
Legionnaires' disease.
Erythromycin, which was previously the drug of choice for pneumonia caused by Legionella pneumophila Legionella micdadei and other Legionella spp, has now been replaced by azithromycin and fluoroquinolones. Azithromycin is highly active against Legionella in vitro, accumulates in tissues in higher concentrations than erythromycin, is easy to use (administered once a day) and is better tolerated (Stout et al., 1998; Garey and Amsden 1999; Yu, 2000). The dose is 500 mg. orally or intravenously for 10-14 days.
Chlamydial infections
. All macrolides are effective against chlamydial infections. Azithromycin is recommended instead of doxycycline for uncomplicated urethritis, endocervicitis, proctitis, and epididymitis (Centers for Disease Control and Prevention, 1998). The main advantage of azithromycin is a single dose, which provides confidence in compliance with the doctor's instructions. For chlamydial infections of the urinary tract and genital organs in pregnant women, the drug of choice is erythromycin (500 mg 4 times a day for 7 days). Azithromycin, 1 g orally as a single dose, can be used instead (Centers for Disease Control and Prevention, 1998). Infants with chlamylia pneumonia and chlamydial conjunctivitis are prescribed erythromycin (50 mg/kg/day in 4 doses for 10-14 days), since tetracyclines are contraindicated for them. For pneumonia caused by Chlamydia pneumoniae, macrolides, fluoroquinolones and tetracyclines are effective. The drugs are prescribed in doses typically used to treat community-acquired pneumonia. No comparative tests have been carried out. The question of the duration of treatment has not been finally resolved. A two-week course is generally recommended (Bartlett et al., 1993). However, in practice, the pathogen is rarely determined and the timing of therapy is established empirically, focusing on clinical effectiveness.
Diphtheria
. Erythromycin allows you to eradicate Corynebacterium diphtheriae from both patients and carriers. In adults, when treated with erythromycin estolate (250 mg 4 times a day for 7 days), carriage can be eliminated in 90% of cases. Other macrolides may also be effective, but experience with them is limited and their use for this purpose is not approved by the FLA. Antibiotics (including erythromycin) do not affect the course of diphtheria and do not reduce the risk of complications; patients are injected with anti-diphtheria serum.
Whooping cough
. Erythromycin is the drug of choice for the treatment of whooping cough and for the prevention of this disease in family members and other persons in close contact with the patient. A seven-day dose of erythromycin estolate (40 mg/kg/day, but not more than 1 g/day) is as effective as the usually recommended two-week course of treatment (Halperin et al., 1997). Clarithromycin and azithromycin appear to be as effective as erythromycin, but experience with them is limited (Aoyama et al., 19%; Vase et al., 1999). Gel treatment was started in the catarrhal period, erythromycin shortens the duration of the disease. Treatment started with the onset of a period of spasmodic cough has almost no effect on the course of the disease, but allows you to eradicate the pathogen from the nasopharynx. If the patient's condition does not improve with antibiotic therapy, culture of material from the nasopharynx is indicated, since there is a report of Bordetella pertussis resistance to erythromycin (Centers for Disease Control, 1994).
Streptococcal infections
. Macrolides are effective for sore throat, pharyngitis, scarlet fever, erysipelas and cellulitis caused by Streptococcus pyogenes, as well as pneumococcal pneumonia. These drugs are prescribed for severe allergies to penicillins. Unfortunately, strains resistant to macrolides are becoming increasingly common. As already noted, penicillin-resistant strains of Streptococcus pneumoniae are often resistant to macrolides.
Staphylococcal infections
. Erythromycin is a reserve drug for the treatment of mild infections caused by both penicillin-sensitive and penicillin-resistant strains of Staphylococcus aureus. However, many strains of Staphylococcus aureus, including community-acquired ones, are resistant to macrolides, so the latter are prescribed only if the sensitivity of the pathogen is confirmed iv vitro.
Infections caused by Campylobacter spp
. For gastroenteritis caused by Campylobacter jejuni, erythromycin (250-500 mg orally 4 times a day for 7 days) accelerates the disappearance of the pathogen from the stool and reduces the duration of the disease (Salazar-Lindo et al., 1986). In adults, erythromycin has been replaced by fluoroquinolones, which are highly active against Campylobacter spp. and other pathogens of intestinal infections. But children are still prescribed erythromycin.
Helicobacter pylori infection
. For peptic ulcers due to infection caused by Helicobacter pylori, clarithromycin (500 mg) is used in combination with omeprazole (20 mg) and amoxicillin (1 g). The drugs are taken 2 times a day for 10-14 days (Peterson et al., 2000). Many other treatment regimens, including 7-day regimens, have been proposed (Misiewicz et al., 1997; Hunt et al., 1999). The most effective ones usually involve 3 drugs, one of which is clarithromycin.
Tetanus
. Erythromycin (500 mg orally every 6 hours for 10 days) is used to eradicate Clostridium tetani in patients with penicillin allergy. However, antibacterial therapy for tetanus plays only a supporting role; The basis of treatment consists of surgical debridement, maintenance of vital functions, administration of antitetanus serum and elimination of seizures.
Syphilis
. Previously, erythromycin was used for early syphilis in patients with allergies to penicillins. Tetracyclines are now used instead of erythromycin (Centers for Disease Control and Prevention, 1998). Pregnant women with allergies to penicillins are recommended to undergo desensitization. Mycobacterial infections. Clarithromycin and azithromycin are the drugs of choice for the prevention and treatment of disseminated infection caused by Mycobacterium avium-intracellulare in patients with SP ID, as well as for the treatment of lung damage caused by this pathogen in patients without HIV infection (American Thoracic Society, 1997; Kovacs and Masur, 2000). For primary prevention of disseminated infection caused by Mycobacterium avium-intracellulare, patients with AIDS with a CD4 lymphocyte count of less than 50 μl are prescribed azithromycin at a dose of 1200 mg once a week or clarithromycin at a dose of 500 mg 2 times a day. Monotherapy is not suitable for the treatment and secondary prevention of this infection in patients with AIDS. In these cases, the treatment of choice is combination treatment with clarithromycin (500 mg 2 times a day) and ethambutol (15 mg/kg 1 time a day); Rifabutin is sometimes prescribed in addition to these two drugs. Azithromycin (500 mg once daily) can be used instead of clarithromycin, but the latter is slightly less effective than clarithromycin (Ward et al., 1998). In combination with minocycline, clarithromycin is used for lepromatous leprosy (Ji et al., 1993).
Other infections
. Clarithromycin and azithromycin are used to treat AIDS-associated toxoplasma encephalitis (Saba et al., 1993) and chronic diarrhea caused by Cryptosporidium spp. (Rehg, 1991). However, the effectiveness of macrolides in these diseases has not been proven in clinical trials. Prevention of rheumatism and infective endocarditis. Erythromycin is used for secondary prevention of rheumatism in patients allergic to penicillins. Previously, such patients were prescribed erythromycin for the prevention of infective endocarditis during dental and respiratory tract interventions. Today, instead of erythromycin, clindamycin is used for this purpose. Clindamycin can be replaced by azithromycin (500 mg once) or clarithromycin (Dajani et al., 1997).
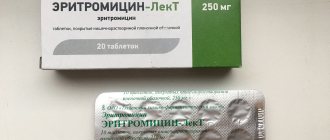
Erythromycin 250 mg No. 20 Tablets
Erythromycin 250 mg No. 20 tablets
Composition active ingredient : erythromycin; 1 tablet contains erythromycin stearate, equivalent to 250 mg or 500 mg of erythromycin; excipients: 250 mg tablet – corn starch, anhydrous colloidal silicon dioxide, sodium starch glycolate (type A), sodium methyl parahydroxybenzoate (E 219), sodium propyl parahydroxybenzoate (E 217), magnesium stearate, talc, rose opadry; tablet 500 mg: microcrystalline cellulose, croscarmellose sodium, povidone, polysorbate 80, colloidal anhydrous silicon dioxide, magnesium stearate, opadry pink. Dosage form Film-coated tablets. Pharmacotherapeutic group Antibacterial agents for systemic use. Macrolides. Erythromycin. ATC code J01F A01. Indications Respiratory tract infections, including atypical pneumonia, infections of the ENT organs (tonsillitis, otitis, sinusitis), purulent-inflammatory diseases of the skin and its appendages, erythrasma, diphtheria, gonorrhea, syphilis, listeriosis, Legionnaires' disease, infections in dentistry and ophthalmology , infections caused by microorganisms resistant to beta-lactam antibiotics, penicillin, tetracyclines, chloramphenicol, streptomycin. Contraindications Hypersensitivity to erythromycin or any other component of the drug, severe liver failure. Simultaneous use with the medications indicated in the relevant section of this instruction - terfenadine, astemizole, cisapride pimozide or ergotamine and dihydroergotamine. Method of administration and dose Set individually, depending on the location and severity of the infection, the sensitivity of the pathogen. Prescribed orally 1-1.5 hours before or 2-3 hours after meals. Adults – 250-500 mg 4 times a day; the highest single dose is 500 mg, the daily dose is 2 g. Children from 3 to 6 years old – 500-700 mg per day; from 6 to 8 years – 700 mg per day; from 8 to 14 years – up to 1 g per day, dividing the daily dose into 4 doses; over 14 years old - in the dose for adults. The course of treatment is 5-14 days; after the symptoms of the disease disappear, the drug is prescribed for another 2 days. Adverse reactions From the digestive tract: nausea, vomiting, epigastric pain, diarrhea, anorexia; rarely – pseudomembranous colitis, pancreatitis. From the hepatobiliary system: impaired liver function, increased levels of “liver” enzymes in the blood serum, hepatocellular or cholestatic hepatitis, cholestatic jaundice. From the senses: hearing loss and/or tinnitus, which disappears after discontinuation of the drug. There have been isolated reports of reversible hearing loss in patients with renal failure who received high doses of erythromycin (more than 4 g/day). From the cardiovascular system: prolongation of the QT interval on the ECG; rarely - ventricular arrhythmias, including ventricular tachycardia and arrhythmia of the torsades de pointes type. From the nervous system: reactions such as confusion, hallucinations, convulsions, dizziness are extremely rare, but their cause-and-effect relationship has not been established. Allergic reactions: skin rashes, itching, urticaria, anaphylactic shock; very rarely - erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis. Other: worsening myasthenia gravis; effects caused by chemotherapy: oral candidiasis, vaginal candidiasis. Overdose Symptoms: nausea, vomiting, diarrhea and discomfort in the stomach; impaired liver function, up to acute liver failure; hearing loss, tinnitus, dizziness (especially in patients with renal or liver failure). Treatment: use of activated carbon, careful monitoring of respiratory status (if necessary, artificial ventilation), acid-base balance and electrolyte metabolism. Gastric lavage is effective when taking a dose 5 times higher than the average therapeutic dose. Hemodialysis, peritoneal dialysis and forced diuresis are ineffective. Use during pregnancy or breastfeeding During pregnancy, use of the drug is possible only when the expected benefit to the mother outweighs the potential risk to the fetus. If it is necessary to prescribe the drug, it is necessary to decide on stopping breastfeeding. Children Used in children over 3 years of age (in this dosage form). Peculiarities of use: When using erythromycin preparations, liver dysfunction has been reported, including increased levels of “liver” enzymes in the blood serum, hepatocellular and/or cholestatic hepatitis with/without jaundice. Erythromycin is eliminated primarily by the liver, so it should be administered with caution to patients with impaired hepatic function, a history of jaundice, or patients being treated with potentially hepatotoxic drugs. During long-term treatment with the drug or when taking large doses, it is necessary to monitor liver function. When treating syphilis in pregnant women, it is necessary to take into account that the drug does not reach therapeutic concentrations in the fetus, therefore, after the birth of the child, penicillin should be prescribed to infants whose mothers used erythromycin. In severely ill patients taking lovastatin concomitantly with erythromycin, cases of rhabdomyolysis with or without renal failure have been observed. Therefore, if it is necessary to prescribe combination treatment with lovastatin or other GABA-CoA reductase inhibitors and erythromycin, it is necessary to carefully assess the benefit/risk ratio, monitor the appearance of symptoms such as muscle pain, weakness, and monitor the levels of creatine kinase and transaminases in the blood serum. Treatment with antibacterial drugs leads to disruption of the normal flora of the large intestine and can cause excessive growth of resistant strains of Clostridium difficile, the toxins of which are the main cause of pseudomembranous colitis. Pseudomembranous colitis occurs both immediately while taking the drug and within 2 months after the end of antibacterial therapy. Cases of mild to life-threatening pseudomembranous colitis have been reported with almost all antibacterial drugs. Therefore, it is important to consider the possibility of developing pseudomembranous colitis in patients with diarrhea after taking antibacterial drugs. In mild cases, it is usually sufficient to discontinue the drug; in severe cases, metronidazole or vancomycin should be prescribed. Taking medications that slow down intestinal motility is contraindicated. When using the drug, it is advisable to determine the causative agent of the disease to reduce the risk of developing resistant forms of bacteria. But treatment with erythromycin can be started before an antibiogram, after which treatment should be continued or an appropriate change in drug should be made. In patients with renal and hepatic insufficiency, in elderly patients, taking into account age-related changes in liver and/or kidney function, the risk of developing an ototoxic effect increases. Elderly patients have an increased risk of developing arrhythmias such as torsades de pointes when treated with erythromycin; The effect of anticoagulation therapy is enhanced when used together with erythromycin. Due to the risk of developing such adverse reactions as prolongation of the QT interval on the ECG, the development of ventricular arrhythmias, including ventricular tachycardia and arrhythmias of the torsades de pointes type, the drug is prescribed with caution to patients with a history of arrhythmias. It is necessary to monitor patients with bronchial asthma taking theophylline and erythromycin, including assessing the level of theophylline in the blood to avoid intoxication. The drug may increase symptoms of the disease in patients with myasthenia gravis. Long-term or repeated use of erythromycin, like other antibacterial drugs, can lead to excessive growth of insensitive microorganisms, in particular fungi. If superinfection develops during treatment, erythromycin should be discontinued and appropriate measures taken. The drug may distort the results of determining the level of catecholamines in urine, carried out using the fluorometric method. Drugs that increase the acidity of gastric juice and acidic drinks inactivate erythromycin. Erythromycin should not be taken with milk or dairy products. The ability to influence the reaction rate when driving vehicles or working with other mechanisms. There are no references to the fact that the drug can negatively affect the reaction rate when driving vehicles or working with other mechanisms. However, due to the possible occurrence of dizziness, caution must be exercised when performing work involving high concentration and when driving vehicles. Interaction with other drugs and other types of interactions The combined use of erythromycin with: astemizole or terfenadine, cisapride, pimozide increases the risk of developing cardiotoxicity: prolongation of the QT interval, severe cardiac arrhythmias, including arrhythmias such as torsades de pointes, cardiac arrest; ergotamine or dihydroergotamine - possible acute toxicity reactions with vasospasm, dysesthesia (erythromycin suppresses the metabolism of ergotamine and dihydroergotamine, increasing vasospasm associated with ergotamine). Biotransformation of erythromycin occurs mainly in the liver with the participation of the cytochrome P450 system. Erythromycin, due to its effect on the activity of cytochrome P450, interacts with the following drugs: increases the concentration of theophylline, aminophylline, caffeine in the blood serum, which increases their toxicity - it is necessary to reduce the doses of these drugs and control their concentration in the blood serum; increases the absorption of digoxin and its concentration in the blood serum; increases the concentration of cyclosporine and increases its nephrotoxicity; is able to reduce the hepatic metabolism of carbamazepine, which makes it possible to reduce the dose of carbamazepine by up to 50% when using drugs simultaneously; increases the concentration and enhances the toxicity of phenytoin, alfentanil, methylprednisolone, benzodiazepines (such as triazolam, alprazolam), hexobarbital, valproic acid, tacrolimus, rifabutin, zopiclone - dose adjustment of these drugs is necessary; with disopyramide, quinidine, procainamide may prolong the QT interval or cause ventricular tachycardia; with oral contraceptives increases the risk of hepatotoxicity; with anticoagulants (warfarin, acetocoumarol) enhances their effects, which are more pronounced in older people. Therefore, prothrombin time should be constantly monitored; increases the blood concentration of GABA-CoA reductase inhibitors (for example, lovastatin, simvastatin) - the risk of rhabdomyolysis increases, which can usually develop after the end of treatment with erythromycin; enhances the systemic effect of sildenafil (Viagra) - it is necessary to reduce the dose of sildenafil; slows down elimination and enhances the effect of calcium channel blockers, such as felodipine, verapamil. There have been reports of hypotension, bradyarrhythmia, and lactic acidosis when taken simultaneously with erythromycin. The effect of the drug is enhanced in combination with sulfonamides, tetracyclines, streptomycin. Colchicine toxicity has been reported when interacting with erythromycin. Do not use together with lincomycin, clindamycin and chloramphenicol (antagonism), with drugs that increase the acidity of gastric juice, as well as with acidic drinks, since they inactivate erythromycin. The drug may affect the results of determining the level of catecholamines in urine using the fluorometric method. Pharmacological properties Pharmacodynamics. Erythromycin is an antibiotic of the macrolide group. The mechanism of action is based on reverse binding to the 50-S subunit of the ribosome in its donor part, blocking the synthesis of proteins in sensitive microbial cells, which leads to disruption of translocation processes and the formation of peptide bonds between amino acid molecules. Erythromycin stearate is stable in an acidic environment, which does not cause destruction of the active substance in the aggressive environment of the digestive tract and improves its bioavailability and tolerability by adults and children. Erythromycin stops the growth and development (causes bacteriostasis) of a number of gram-positive bacteria: Staphylococcus aureus, Streptococcus pyogenes (beta-hemolytic streptococcus of group A), alpha-hemolytic streptococcus (Viridans group), Streptococcus pneumoniae, Corynebacterium diphtheriae, Corynebacterium minutissimum, gram-negative bacteria: Neisseria gonorrhoeae , Legionella pneumophila, Bordetella pertussis and some other microorganisms: Mycoplasma pneumoniae, Ureaplasma urealyticum, Chlamydia trachomatis, Entamoeba histolytica, Treponema pallidum, Listeria monocytogenes. Gram-negative bacilli are insensitive: Escherichia coli, Pseudomonas aeruginosa, as well as Shigella and Salmonella. Pharmacokinetics. It is well absorbed in the digestive tract; gastric contents and an acidic environment somewhat slow down the absorption process, but not the activity of the active substance. Foods high in carbohydrates, fats and proteins increase the absorption of erythromycin stearate by 53-64%. It is hydrolyzed in the duodenum, where it is absorbed. The maximum concentration in blood plasma is achieved 2 hours after enteral administration. The binding to serum proteins is variable (from 73% for erythromycin to 93% for erythromycin stearate). Penetrates well into body cavities (in pleural, peritoneal and synovial fluids the concentration is 15-30% of that in the blood), and in muscle tissue, prostate and seminal fluid in concentrations equal to those of sirovatkov. Diffuses into poorly vascularized organs and tissues (concentration in the middle ear is 50% of the serum concentration). It practically does not penetrate through the intact blood-brain barrier; passes through the placental barrier and is excreted into breast milk. Erythromycin is distributed within a total absorption volume of 0.75 L/kg and has a half-life of 1-1.5 hours. It undergoes biotransformation in the liver with the formation of inactive metabolites. It is excreted mainly with bile, with 20-30% in active form and 5-15% unchanged in urine. The pharmacokinetics of erythromycin stearate in infants, children, adults and the elderly are not significantly different. Basic physicochemical properties of 250 mg tablets – round, biconvex, film-coated tablets, pink in color; 500 mg tablets are oval, biconvex tablets, pink coated. Shelf life: 3 years. Storage conditions Store in the original packaging, out of reach of children at a temperature not exceeding 25 °C. Packaging Film-coated tablets, 250 mg: 10 tablets in a blister, 2 blisters in a pack. Film-coated tablets, 500 mg: 10 tablets in a blister, 1 blister in a pack. Vacation category: Prescription.
Side effects[edit | edit code]
Erythromycin rarely causes severe side effects. Allergic reactions include fever, eosinophilia, and rash, which may occur alone or in combination. After discontinuation of the drug, the symptoms quickly disappear. The most severe side effect is cholestatic hepatitis. It is mainly caused by erythromycin estolate, very rarely by erythromycin ethylsuccinate or erythromycin stearate (Ginsburg and Eichenwald, 1976). The disease begins approximately 10-20 days after the start of treatment with nausea, vomiting and cramping abdominal pain. The pain is often the same as with | acute cholecystitis, which may lead to unnecessary surgery. Jaundice soon appears, sometimes accompanied by fever, leukocytosis, eosinophilia and increased aminotransferase activity. Liver biopsy reveals cholestasis, periportal infiltration of neutrophils, lymphocytes and eosinophils, and sometimes necrosis of hepatocytes. Manifestations of hepatitis rarely persist for more than a few days after discontinuation of the drug. It is possible that cholestatic hepatitis results from an allergic reaction to erythromycin estolate (Tolman et al., 1974). There may be a slight increase in serum liver enzyme activity (McCormack et al., 1977).
When taken orally, especially in large doses, erythromycin often causes epigastric pain, sometimes quite severe. With intravenous administration, gastrointestinal disorders are also possible - cramping abdominal pain, nausea, vomiting, diarrhea. Erythromycin has been shown to enhance gastrointestinal motility by binding to motilin receptors (Smith et al., 2000). Gastrointestinal disturbances are dose-dependent and are more common in children and young adults (Seifert et al., 1989). Longer infusion (over 1 hour) and pre-administration of glycopyrronium bromide alleviate these symptoms (Bowler et al., 1992). IV administration of the drug at a dose of 1 g, even when diluted in a large volume of liquid, is often accompanied by thrombophlebitis. Slow administration reduces the risk of this complication.
There are reports that erythromycin causes cardiac arrhythmias, including prolongation of the QT interval and, against its background, ventricular tachycardia. In most cases, these disorders occurred in patients with heart disease or were observed when erythromycin was prescribed concomitantly with drugs such as cisapride and terfenadine (Brandriss et al., 1994).
The use of erythromycin in large doses (erythromycin glucoheptonate or erythromycin lactobionate, 4 g/day IV, or large doses of erythromycin estolate orally) may be accompanied by transient hearing loss (Karmody and Weinstein, 1977).
Interactions and side effects
Erythromycin is metabolized by cytochrome P450 3A4, 3A5, 3A7. Therefore, the biotransformation of substances that are metabolized by these same enzymes may be slowed down. These substances include, for example, cyclosporine, diazepam, lidocaine, warfarin. When used together with erythromycin, there is a possibility of their excessive accumulation in the body.
Erythromycin is well tolerated by the human body . The most common side effects are mild gastrointestinal disorders. Erythromycin is a powerful prokinetic agent that accelerates gastric emptying. Very rarely, allergic reactions, tinnitus, and temporary hearing loss may occur.
Drug interactions[edit | edit code]
Erythromycin and clarithromycin interact with other drugs (Periti et al., 1992). Erythromycin enhances the effects of astemizole, carbamazepine, glucocorticoids, cyclosporine, digoxin, ergot alkaloids, terfenadine, theophylline, triazolam, valproic acid and warfarin, probably by inhibiting liver microsomal enzymes involved in the metabolism of these drugs (Ludden, 1985; Martell et al. , 1986; Honig et al., 1992). Clarithromycin, which is similar in structure to erythromycin, interacts with the same drugs. Azithromycin, apparently, does not enter into drug interactions, since, unlike erythromycin and clarithromycin, it contains a 15-membered lactone ring. However, azithromycin should be prescribed concomitantly with the drugs listed above with caution.
Read also[edit | edit code]
- Antibiotics (antimicrobial agents) Choice of antibiotic
- Combination antibiotic therapy
- Prophylactic antibiotic therapy
- Mechanisms of action of antibiotics
- Sulfonamides, trimethoprim/sulfamethoxazole
- Quinolones and urinary antiseptics
- Beta-lactam antibiotics Penicillins
- Cephalosporins
- Carbapenems
- Beta-lactamase inhibitors
- Tetracyclines
- Antituberculosis drugs (antimycobacterial) Isoniazid
- Rifampicin
- Ethambutol
- Streptomycin
- Pyrazinamide
- Other anti-tuberculosis drugs