Indications and spectrum of action
Clarithromycin is used to treat bacterial infections of the respiratory system (such as bronchitis or pneumonia), ear, nose, and throat (such as tonsillitis or sinusitis), and skin (such as impetigo or erysipelas).
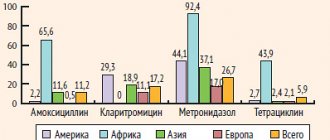
Clarithromycin is active against streptococci (including pneumococci), legionella, chlamydia, bacteria without a cell wall (for example mycoplasma), atypical mycobacteria, as well as Bordetella pertussis.
The clinical effectiveness of all macrolides against Haemophilus influenzae is currently considered insufficient.
Clarithromycin is also used in combination therapy to destroy Helicobacter pylori colonies in the gastric mucosa (gastritis).
Description
Capsule-shaped biconvex tablets, pink film-coated. On a cross section, two layers are visible, the inner layer is white or almost white.
Pharmacological group:
antibiotic - macrolide.
ATX code:
J01FA09.
Pharmacological properties:
Pharmacodynamics. Semi-synthetic broad-spectrum macrolide antibiotic. Disturbs the protein synthesis of microorganisms (binds to the 50S subunit of the ribosomes of the microbial cell). Acts on externally and intracellularly located pathogens. The activity of clarithromycin against most of the following microorganisms has been proven in vitro and in clinical practice - aerobic gram-positive microorganisms: Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes; aerobic gram-negative microorganisms: Haemophilus influenzae, Haemophilus parainfluenzae, Moraxella catarrhalis, Legionella pneumophila; other microorganisms: Mycoplasma pneumoniae, Chlamydia pneumoniae; mycobacteria: Mycobacterium avium complex (MAC) - a complex including: Mycobacterium avium and Micobacterium intracellulare; Helycobacter pylori.
Beta-lactamases do not affect the activity of clarithromycin.
Activity of clarithromycin in vitro - aerobic gram-positive microorganisms: Listeria monocytogenes, Streptococcus agalactiae, Streptococci groups C,F,G, Streptococci group viridans; aerobic gram-negative microorganisms: Neisseria gonorrhoeae, Bordetella pertussis, Pasteurella multocida; anaerobic gram-positive microorganisms: Clostridium perfringens, Peptococcus niger, Propionibacterium acnes; anaerobic gram-negative microorganisms: Bacteroides melaninogenicus; spirochetes: Borrelia burgdorferi, Treponema pallidum; mycobacteria: Mycobacterium leprae, Mucobacterium chelonae, campylobacteria: Campylobacter jejuni.
The microbiologically active metabolite of clarithromycin, 14-hydroxyclarithromycin, is twice as active as the parent compound against Haemophilus influenzae. Clarithromycin and its metabolite in combination may have either additive or synergistic effects on Haemophilus influenzae in vitro and in vivo, depending on the strain of the bacterium.
Most strains of staphylococci resistant to methicillin and oxacillin are resistant to clarithromycin.
It is possible to develop cross-resistance to clarithromycin and other macrolide antibiotics, as well as lincomycin and clindamycin.
Pharmacokinetics
Absorption is fast. Food slows absorption without significantly affecting bioavailability. Bioavailability of 250 mg tablets is 50%. Bonding with plasma proteins is 65-75%. After a single dose, two peaks of maximum concentration (Cmax) are recorded. The second peak is due to the ability of the drug to accumulate in the gallbladder, followed by gradual or rapid entry into the intestine and absorption. The time to reach maximum concentration (TCmax) when taking 250 mg is 2-3 hours.
After oral administration, 20-30% of the dose taken is quickly hydroxylated in the liver by cytochrome CYP3A4, CYP3A5 and CYP3A7 isoenzymes to form the main metabolite - 14-hydroxyclarithromycin, which has pronounced antimicrobial activity against Haemophllus influenzae. It is an inhibitor of the CYP3A4, CYP3A5 and CYP3A7 isoenzymes.
When taken regularly at 250 mg/day, the equilibrium concentration (Css) of the unchanged drug and its main metabolite is 1 and 0.6 μg/ml, respectively; half-life is 3-4 and 5-6 hours, respectively. When the dose is increased to 500 mg/day, Css of the unchanged drug and its metabolite in plasma is 2.7-2.9 and 0.83-0.88 μg/ml, respectively; half-life is 4.8-5 and 6.9-8.7 hours, respectively. At therapeutic concentrations it accumulates in the lungs, skin and soft tissues (their concentrations are 10 times higher than the level of the antibiotic in the blood plasma).
It is excreted by the kidneys and intestines (20-30% in unchanged form, the rest in the form of metabolites). With a single dose of 250 and 1200 mg, 37.9 and 46% are excreted by the kidneys, and 40.2 and 29.1% by the intestines, respectively.
If renal function is impaired, there is an increase in TCmax, Cmax and the area under the concentration-time curve (AUC) of clarithromycin and its metabolite.
Contraindications
Due to the predominance of metabolism in the liver, the use of clarithromycin is contraindicated in patients with severe hepatic impairment.
The use of clarithromycin is contraindicated if there is hypersensitivity to clarithromycin or other macrolide antibiotics (for example, azithromycin).
General use with cisapride, pimozide, terfenadine, or astemizole is prohibited because life-threatening arrhythmias may occur.
In addition, clarithromycin should not be taken with the ergot alkaloids dihydroergotamine and ergotamine. Combined use may lead to intoxication and vasospasm.
Indications for use:
Adults: pharyngitis, tonsillitis, acute sinusitis, exacerbation of chronic bronchitis, community-acquired pneumonia, uncomplicated infections of the skin and subcutaneous tissue; disseminated infection caused by Mycobacteiium avium and Mycobacterium intracellulare.
Adults in combination with amoxicillin and omeprazole/lansoprazole as triple therapy for infections caused by Helicobacter pylori, including duodenal ulcer.
Children: pharyngitis, tonsillitis, community-acquired pneumonia, acute sinusitis, acute otitis media, uncomplicated infections of the skin and subcutaneous tissue; disseminated infection caused by Mycobacterium avium and Mycobacterium intracellulare.
Interactions with other drugs
Because clarithromycin inhibits the activity of the cytochrome P450 isoenzyme CYP3A4, the metabolism of many other drugs may be slowed. As a result, their concentration increases and the effect intensifies. There are a huge variety of such drugs, here are the most common examples:
- quinidine and disopyramide,
- anticonvulsant carbamazepine,
- anti-gout drug - colchicine,
- cardiac glycoside - digoxin,
- statins,
- anticoagulants,
- sildenafil, tadalafil and vardenafil,
- theophylline,
- tolterodine,
- benzodiazepines (triazolbenzodiazepines, e.g. midazolam, triazolam, alprazolam),
- antivirals - zidovudine, atazanavir, saquinavir,
- proton pump blocker omeprazole,
- calcium antagonists,
- itraconazole and others.
Concomitant use of all these drugs with clarithromycin should be carefully monitored.
Clarithromycin
The simultaneous use of clarithromycin and the following drugs is contraindicated due to the possibility of serious side effects.
Cisapride, pimozide, terfenadine and astemizole
When clarithromycin was taken together with cisapride, pimozide, terfenadine, astemizole, an increase in the concentration of the latter in the blood plasma was reported, which can lead to an increase in the QT interval and the appearance of cardiac arrhythmias, including ventricular tachycardia, ventricular fibrillation and torsade de pointes (see section "Contraindications").
Ergot alkaloids
When clarithromycin is used together with ergotamine or dihydroergotamine, the following effects associated with acute poisoning with drugs of the ergotamine group are possible: vascular spasm, ischemia of the limbs and other tissues, including the central nervous system. The simultaneous use of clarithromycin and ergot alkaloids is contraindicated (see section "Contraindications").
HMG-CoA reductase inhibitors (statins)
Co-administration of clarithromycin with lovastatin or simvastatin is contraindicated (see section "Contraindications") due to the fact that these statins are largely metabolized by the CYP3A4 isoenzyme, and combined use with clarithromycin increases their serum concentrations, which leads to an increased risk of developing myopathy, including Rhabdomyolysis Cases of rhabdomyolysis have been reported in patients taking clarithromycin concomitantly with these drugs. If clarithromycin is necessary, lovastatin or simvastatin should be discontinued during therapy.
Clarithromycin should be used with caution in combination therapy with statins. It is recommended to use statins that do not depend on CYP3A metabolism (for example, fluvastatin). If coadministration is necessary, it is recommended to take the lowest dose of statin. The development of signs and symptoms of myopathy should be monitored.
Effect of other drugs on clarithromycin
Drugs that are CYP3A inducers (for example, rifampicin, phenytoin, carbamazepine, phenobarbital, St. John's wort) may induce the metabolism of clarithromycin. This may result in subtherapeutic concentrations of clarithromycin, resulting in reduced effectiveness. In addition, it is necessary to monitor the concentration of the CYP3A inducer in the blood plasma, which may increase due to inhibition of the CYP3A isoenzyme by clarithromycin. When rifabutin and clarithromycin were used together, an increase in plasma concentrations of rifabutin and a decrease in serum concentrations of clarithromycin were observed with an increased risk of developing uveitis.
The following drugs have a proven or suspected effect on clarithromycin plasma concentrations; if used concomitantly with clarithromycin, dosage adjustments or switching to alternative treatment may be required.
Efavirenz, nevirapine, rifampicin, rifabutin and rifapentine
Strong inducers of the cytochrome P450 system, such as efavirenz, nevirapine, rifampicin, rifabutin and rifapentine, can accelerate the metabolism of clarithromycin and, thus, reduce the plasma concentration of clarithromycin and weaken the therapeutic effect, and at the same time increase the concentration of the 14-OH-clarithromycin metabolite, also being microbiologically active. Since the microbiological activity of clarithromycin and 14-OH-clarithromycin differs against different bacteria, the therapeutic effect may be reduced when clarithromycin is used together with enzyme inducers.
Etravirine
The concentration of clarithromycin decreases with the use of etravirine, but the concentration of the active metabolite 14-OH-clarithromycin increases. Because 14-OP-clarithromycin has low activity against Mycobacterium avium complex (MAC) infections, overall activity against these pathogens may be affected, and alternative treatments should be considered for the treatment of MAC.
Fluconazole
Coadministration of fluconazole 200 mg daily and clarithromycin 500 mg twice daily in 21 healthy volunteers resulted in an increase in mean clarithromycin minimum steady-state concentration (Cmin) and AUC by 33% and 18%, respectively. However, co-administration did not significantly affect the average steady-state concentration of the active metabolite 14-OH-clarithromycin. No dose adjustment of clarithromycin is required when taking fluconazole concomitantly.
Ritonavir
Coadministration of ritonavir 200 mg every eight hours and clarithromycin 500 mg every 12 hours resulted in a marked suppression of the metabolism of clarithromycin. When co-administered with ritonavir, clarithromycin Cmax increased by 31%, Cmin increased by 182% and AUC increased by 77%. Complete suppression of the formation of 14-OH-clarithromycin was noted. Due to the wide therapeutic range of clarithromycin, dose reduction is not required in patients with normal renal function. In patients with renal failure, it is advisable to consider the following dose adjustment options: with creatinine clearance of 30-60 ml/min, the dose of clarithromycin should be reduced by 50%; if creatinine clearance is less than 30 ml/min, the dose of clarithromycin should be reduced by 75%. Ritonavir should not be co-administered with clarithromycin in doses exceeding 1 g/day.
Effect of clarithromycin on other drugs Antiarrhythmic drugs (quinidine and disopyramide)
Ventricular tachycardia of the “pirouette” type may occur with the combined use of clarithromycin and quinidine or disopyramide. When clarithromycin is coadministered with these drugs, the electrocardiogram should be regularly monitored for prolongation of the QT interval, and serum concentrations of these drugs should also be monitored. During post-marketing use, cases of hypoglycemia have been reported during co-administration of clarithromycin and disopyramide. It is necessary to monitor the concentration of glucose in the blood while using clarithromycin and disopyramide.
Oral hypoglycemic agents and insulin
When clarithromycin is used together with oral hypoglycemic agents (for example, sulfonylureas) and/or insulin, severe hypoglycemia may occur. During concomitant use of clarithromycin and certain drugs that lower glucose concentrations, such as nateglinide, pioglitazone, repaglinide and rosiglitazone, inhibition of the CYP3A isoenzyme by clarithromycin may occur, which may result in hypoglycemia. Careful monitoring of glucose concentrations is recommended.
Interactions due to CYP 3 A
Co-administration of clarithromycin, which is known to inhibit the CYP3A isoenzyme, and drugs primarily metabolized by the CYP3A isoenzyme, may be associated with a mutual increase in their concentrations, which may increase or prolong both therapeutic and side effects. Clarithromycin should be used with caution in patients receiving drugs that are substrates of the CYP3A isoenzyme, especially if these drugs have a narrow therapeutic index (for example, carbamazepine) and/or are extensively metabolized by this enzyme. If necessary, the dose of the drug taken together with clarithromycin should be adjusted. Also, whenever possible, serum concentrations of drugs primarily metabolized by CYP3A should be monitored.
The following drugs/classes are metabolized by the same CYP3A isoenzyme as clarithromycin, for example, alprazolam, carbamazepine, cilostazol, cyclosporine, disopyramide, methylprednisolone, midazolam, omeprazole, indirect anticoagulants (eg, warfarin), quinidine, rifabutin, sildenafil, tacrolimus, triazolam and vinblastine. Also, agonists of the CYP3A isoenzyme include the following drugs that are contraindicated for combined use with clarithromycin: astemizole, cisapride, pimozide, terfenadine, lovastatin, simvastatin and ergot alkaloids (see section “Contraindications”). Drugs that interact in this manner through other isoenzymes within the cytochrome P450 system include phenytoin, theophylline, and valproic acid. Co-administration of clarithromycin, which is known to inhibit the CYP3A4 isoenzyme, and quetiapine, which is a CYP3A4 substrate, may lead to increased exposure of the antipsychotic quetiapine and possible toxic effects.
There have been post-marketing reports of somnolence, orthostatic hypotension, altered states of consciousness, neuroleptic malignant syndrome, and QT prolongation when these drugs are used together.
Caution should be used when quetiapine is used in combination with CYP3A4 inhibitors such as clarithromycin. The dose of quetiapine may need to be reduced.
Indirect anticoagulants
When taking warfarin and clarithromycin together, bleeding and a marked increase in the international normalized ratio (INR) and prothrombin time are possible. In case of combined use with warfarin or other indirect anticoagulants, it is necessary to monitor the INR and prothrombin time.
Omeprazole
Clarithromycin (500 mg every 8 hours) was studied in healthy adult volunteers in combination with omeprazole (40 mg daily). When clarithromycin and omeprazole were used together, steady-state plasma concentrations of omeprazole were increased (Cmax, AUCo-24 and T1/2 increased by 30%, 89% and 34%, respectively). The mean 24-hour gastric pH was 5.2 when omeprazole was taken alone and 5.7 when omeprazole was taken with clarithromycin.
Sildenafil, tadalafil and vardenafil
Each of these phosphodiesterase-5 inhibitors is metabolized at least in part by CYP3A. However, CYP3A may be inhibited in the presence of clarithromycin. Concomitant use of clarithromycin with sildenafil, tadalafil or vardenafil may result in increased phosphodiesterase inhibitory effects. When using these drugs together with clarithromycin, consider reducing the dose of sildenafil, tadalafil and vardenafil.
Theophylline, carbamazepine
When clarithromycin and theophylline or carbamazepine are used together, the concentration of these drugs in the systemic circulation may increase.
Tolterodine
The primary metabolism of tolterodine occurs through the 2D6 isoform of cytochrome P450 (CYP2D6). However, in part of the population lacking the CYP2D6 isoenzyme, metabolism occurs through CYP3A. In this population, inhibition of CYP3A results in significantly higher serum concentrations of tolterodine. In populations that are poor metabolizers via CYP2D6, a reduced dose of tolterodine may be required in the presence of CYP3A inhibitors such as clarithromycin.
Benzodiazepines (eg, alprazolam, midazolam, triazolam)
When midazolam was co-administered with clarithromycin tablets (500 mg twice daily), midazolam AUC increased by 2.7 times after intravenous midazolam and 7 times after oral administration. Concomitant oral administration of midazolam and clarithromycin should be avoided. Concomitant use of clarithromycin with oral midazolam is contraindicated. If intravenous midazolam is used concomitantly with clarithromycin, the patient's condition should be carefully monitored for possible dose adjustment. The same precautions should be applied to other benzodiazepines that are metabolized by CYP3A, including triazolam and alprazolam. For benzodiazepines whose elimination is not dependent on CYP3A (temazepam, nitrazepam, lorazepam), a clinically significant interaction with clarithromycin is unlikely.
When clarithromycin and triazolam are used together, central nervous system (CNS) effects such as drowsiness and confusion are possible. Therefore, if coadministration occurs, it is recommended to monitor for symptoms of CNS impairment.
Interactions with other drugs
Aminoglycosides
When taking clarithromycin concomitantly with other ototoxic drugs, especially aminoglycosides, caution should be exercised and the functions of the vestibular and auditory systems should be monitored both during and after therapy.
Colchicine
Colchicine is a substrate of both CYP3A and the P-glycoprotein (Pgp) transporter protein. Clarithromycin and other macrolides are known to be inhibitors of CYP3A and Pgp. When clarithromycin and colchicine are taken together, inhibition of Pgp and/or CYP3A may result in increased effects of colchicine. The development of clinical symptoms of colchicine poisoning should be monitored. There have been post-marketing reports of cases of colchicine poisoning when taken concomitantly with clarithromycin, most often in elderly patients. Some of the reported cases occurred in patients suffering from kidney failure. Some cases were reported to be fatal. The simultaneous use of clarithromycin and colchicine is contraindicated (see section "Contraindications").
Digoxin
Digoxin is suspected to be a Pgp substrate. Clarithromycin is known to inhibit Pgp. When clarithromycin and digoxin are co-administered, inhibition of Pgp by clarithromycin may result in increased effects of digoxin. Coadministration of digoxin and clarithromycin may also result in increased serum concentrations of digoxin. Some patients have experienced clinical symptoms of digoxin toxicity, including potentially fatal arrhythmias. When clarithromycin and digoxin are used together, serum digoxin concentrations should be carefully monitored.
Zidovudine
Concomitant use of clarithromycin tablets and oral zidovudine in adult HIV-infected patients may result in decreased steady-state zidovudine concentrations.
Because clarithromycin interferes with the oral absorption of zidovudine, the interaction can be largely avoided by taking clarithromycin and zidovudine 4 hours apart.
This interaction was not observed in HIV-infected children taking clarithromycin pediatric suspension with zidovudine or dideoxyinosine. Since clarithromycin may interfere with the absorption of zidovudine when administered concomitantly orally in adult patients, such an interaction is unlikely to occur when clarithromycin is used intravenously.
Phenytoin and valproic acid
There is evidence of interactions between CYP3A inhibitors (including clarithromycin) and drugs that are not metabolized by CYP3A (phenytoin and valproic acid). For these drugs, when used together with clarithromycin, it is recommended to determine their serum concentrations, as there are reports of their increase.
Bidirectional drug interactions
Atazanavir
Clarithromycin and atazanavir are both substrates and inhibitors of the CYP3A isoenzyme. There is evidence of a bidirectional interaction between these drugs. Coadministration of clarithromycin (500 mg twice daily) and atazanavir (400 mg once daily) may result in a twofold increase in clarithromycin exposure and a 70% decrease in 14-OH-clarithromycin exposure, with a 28% increase in atazanavir AUC. Due to the wide therapeutic range of clarithromycin, dose reduction is not required in patients with normal renal function. In patients with moderate renal failure (creatinine clearance 30 - 60 ml/min), the dose of clarithromycin should be reduced by 50%. In patients with creatinine clearance less than 30 ml/min, the dose of clarithromycin should be reduced by 75% using the appropriate clarithromycin dosage form. Clarithromycin in doses exceeding 1000 mg per day should not be used in conjunction with protease inhibitors.
Blockers of "slow" calcium channels
When using clarithromycin simultaneously with blockers of “slow” calcium channels that are metabolized by the CYP3A4 isoenzyme (for example, verapamil, amlodipine, diltiazem), caution should be exercised as there is a risk of arterial hypotension. Plasma concentrations of clarithromycin, as well as slow calcium channel blockers, may increase with simultaneous use. Arterial hypotension, bradyarrhythmia and lactic acidosis are possible when taking clarithromycin and verapamil simultaneously.
Itraconazole
Clarithromycin and itraconazole are substrates and inhibitors of the CYP3A isoenzyme, which determines the bidirectional interaction of the drugs. Clarithromycin may increase plasma concentrations of itraconazole, while itraconazole may increase plasma concentrations of clarithromycin. Patients taking itraconazole and clarithromycin concomitantly should be closely monitored for symptoms of increased or prolonged pharmacological effects of these drugs.
Saquinavir
Clarithromycin and saquinavir are CYP3A substrates and inhibitors, resulting in a bidirectional drug interaction. Coadministration of clarithromycin (500 mg twice daily) and saquinavir (soft gelatin capsules, 1200 mg three times daily) in 12 healthy volunteers increased the AUC and Cmax of saquinavir by 177% and 187%, respectively, compared with saquinavir. separately. The AUC and Cmax values of clarithromycin were approximately 40% higher than with clarithromycin monotherapy. When these two drugs are used together for a limited time at the doses/dosage forms indicated above, no dose adjustment is required. Results from drug interaction studies using saquinavir soft gelatin capsules may not be consistent with the effects observed with saquinavir hard gelatin capsules. The results of drug interaction studies with saquinavir monotherapy may not be consistent with the effects observed with saquinavir hard gelatin capsules. The results of drug interaction studies with saquinavir monotherapy may not be consistent with the effects observed with saquinavir/ritonavir therapy. When taking saquinavir with ritonavir, consider the potential effect of ritonavir on clarithromycin.
Side effects
The most common side effects from the gastrointestinal tract are nausea, vomiting, epigastric discomfort, colic, soft and dark stools, and diarrhea. If severe and persistent diarrhea occurs, pseudomembranous colitis should be suspected. Other common side effects include decreased sense of smell or taste. Sometimes hypersensitivity reactions may occur.
Rare side effects include short-term cholestasis and jaundice. In critically ill patients with relevant comorbidities, the risk of death may increase. Also a rare side effect is a change in myocardial excitability, which is manifested on the ECG by an increase in the QT interval and tachycardia. This fact increases the risk of life-threatening arrhythmias.
Side effects related to the central nervous system are rare. These include dizziness, headache, anxiety, drowsiness, hallucinations, nightmares, and psychosis. Also rare are inflammation and discoloration of the tongue, inflammation of the gums and/or oral mucosa, fungal infections of the mucous membranes, and tooth discoloration. Tooth discoloration can be eliminated by cleaning.
Very rare: pancreatitis, interstitial nephritis, leukopenia, thrombocytopenia.
Use during pregnancy and lactation:
The safety of clarithromycin during pregnancy has not been established. During pregnancy, especially in the first trimester, it is recommended to prescribe clarithromycin if the benefits of its use outweigh the potential risks to the fetus and/or there is no safer alternative therapy. If pregnancy occurs while taking the drug, the patient should be warned about the possible risks to the fetus. If it is necessary to prescribe the drug during lactation, the issue of stopping breastfeeding should be resolved.
Clarithromycin recipe in Latin
The Latin prescription for clarithromycin is prescribed in the form of tablets with a dosage of 250 and 500 mg; in the form of extended-release (ER) tablets 500 mg; in the form of a suspension for oral administration 125 or 250 mg/5 ml; in bottles in powder form 500 mg.
Read the general rules for writing a prescription in Latin here.
For example, let's prescribe clarithromycin at a dosage of 250 mg in tablet form - read more about the recipe for tablets in Latin.
Rp.: Clarithromycini 250 mg Dtd N 14 in tab. S. Orally, 1 tablet 2 times a day.
We will also write out a prescription for clarithromycin in Latin in the form of a suspension with a dosage of 250 mg/5 ml - more details about the recipe for the suspension in Latin. Since the suspensions are produced in bottles, there is no need to indicate them - for more details, see the recipe for the bottles in Latin.
Rp.: Susp. Clarithromycini 250 mg/5 ml Dtd N 10 S. Orally, 1 suspension 2 times a day.
The recipe for clarithromycin in Latin in powder form, more details about the recipe for powder in Latin, will look like this:
Rp.: Pulv. Clarithromycini 0.5 Dtd N 12 S. Dilute with saline. solution, intramuscularly 1 time per day.
Compound:
Each tablet contains:
Active substance:
clarithromycin (in terms of active substance) 250.0 mg; 500.0 mg
| Excipients: | ||||
| 300.0 mg | 600.0 mg | |||
| povidone-K25 | 9.1 mg | 18.2 mg | ||
| magnesium stearate | 6.5 mg | 13.0 mg | ||
| silicon dioxide colloidal (aerosil) | 4.33 mg | 8.66 mg | ||
| talc | 13.0 mg | 26.0 mg | ||
| polacrilin potassium | until you get an uncoated tablet weighing | |||
| 650.0 mg | 1300.0 mg | |||
| Shell excipients: | ||||
| hypromellose | 9.52 mg | 14.28 mg | ||
| talc | 1.14 mg | 1.71 mg | ||
| titanium dioxide | 5.171 mg | 7.756 mg | ||
| macrogol-4000 | 3.726 mg | 5.589 mg | ||
| povidone-K17 | 0.414 mg | 0.621 mg | ||
| Azorubine dye | 0.029 mg | 0.044 mg | ||
| before obtaining a coated tablet mass | ||||
| 670.0 mg | 1330.0 mg | |||
Special instructions:
In the presence of chronic liver diseases, it is necessary to regularly monitor the activity of enzymes in the blood serum.
Prescribe with caution against drugs metabolized by the liver (it is recommended to measure their concentration in the blood).
In case of co-administration with warfarin or other anticoagulants, it is necessary to monitor the prothrombin time.
If a secondary infection develops, adequate therapy should be prescribed.
If severe diarrhea occurs during or after treatment, the diagnosis of pseudomembranous colitis should be excluded, which requires immediate discontinuation of the drug and the appointment of appropriate treatment.
Directions for use and dosage:
Take orally, swallow the tablets without chewing, with a small amount of liquid.
Adults and children over 12 years of age and weighing more than 33 kg:
- for pharyngitis and tonsillitis caused by Streptococcus pyogenes - 250 mg every 12 hours for 10 days;
- for acute sinusitis, 500 mg every 12 hours for 14 days;
- for exacerbation of chronic bronchitis caused by Haemophilus influenzae - 500 mg every 12 hours for 7-14 days, caused by Haemophilus parainfluenzae - 500 mg every 12 hours for 7 days; caused by Moraxella catarrhalis, Streptococcus pneumoniae - 250 mg every 12 hours for 7-14 days;
- for community-acquired pneumonia caused by Haemophilus influenzae - 250 mg every 12 hours for 7 days, caused by Streptococcus pneumoniae, Chlamydia pneumoniae, Mycoplasma pneumoniae - 250 mg every 12 hours for 7-14 days;
- for uncomplicated infections of the skin and subcutaneous tissue caused by Staphylococcus aureus, Streptococcus pyogenes - 250 mg every 12 hours for 7-14 days.
In the treatment and prevention of infections caused by Mycobacterium avium
prescribed 500 mg 2 times a day. The maximum daily dose is 1000 mg. Duration of treatment – 6 months. and more.
To eradicate Helicobacter pylori:
Combined treatment with three drugs:
clarithromycin - 500 mg, lansoprazole - 30 mg and amoxicillin - 1000 mg 2 times a day for 10-14 days;
clarithromycin - 500 mg, omeprazole - 20 mg and amoxicillin - 1000 mg 2 times a day for 10 days.
Combined treatment with two drugs:
Clarithromycin - 500 mg 3 times a day, omeprazole - 40 mg per day for 14 days, with the prescription of omeprazole for the next 14 days at a dose of 20 mg per day.
For patients with chronic renal failure:
(creatinine clearance less than 30 ml/min or serum creatinine concentration more than 3.3 mg/100 ml), the dose is reduced by 2 times, or the interval is increased by 2 times. The maximum duration of treatment for patients in this group is 14 days.