Nosological classification (ICD-10)
- A48.3 Toxic shock syndrome
- A49 Bacterial infection of unspecified localization
- C71 Malignant neoplasm of the brain
- C80 Malignant neoplasm without specification of localization
- C82 Follicular [nodular] non-Hodgkin's lymphoma
- C83 Diffuse non-Hodgkin's lymphoma
- C84 Peripheral and cutaneous T-cell lymphomas
- C85 Other and unspecified types of non-Hodgkin's lymphoma
- C95 Leukemia of unspecified cell type
- C95.0 Acute leukemia of unspecified cell type
- C95.9 Leukemia, unspecified
- D59 Acquired hemolytic anemia
- D69.3 Idiopathic thrombocytopenic purpura
- D70 Agranulocytosis
- E06.1 Subacute thyroiditis
- E27.1 Primary adrenal insufficiency
- E27.2 Addison's crisis
- E27.4 Other and unspecified adrenal insufficiency
- E83.5.0* Hypercalcemia
- G00 Bacterial meningitis, not elsewhere classified
- G04 Encephalitis, myelitis and encephalomyelitis
- G93.6 Cerebral edema
- H01.0 Blepharitis
- H10.1 Acute atopic conjunctivitis
- H10.5 Blepharoconjunctivitis
- H15.0 Scleritis
- H15.1 Episcleritis
- H16.2 Keratoconjunctivitis
- H16.8 Other forms of keratitis
- H20.9 Iridocyclitis, unspecified
- H45.1 Endophthalmitis in diseases classified elsewhere
- I61 Intracerebral hemorrhage
- J40 Bronchitis, not specified as acute or chronic
- J44 Other chronic obstructive pulmonary disease
- J45 Asthma
- J46 Status asthmaticus
- J98.8.0* Bronchospasm
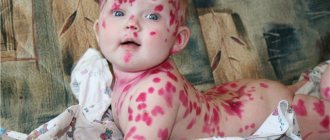
- L30.9 Dermatitis, unspecified
- L91.0 Keloid scar
- L92.0 Granuloma annulare
- L93.0 Discoid lupus erythematosus
- M35.9 Systemic connective tissue disorders, unspecified
- M79.0 Rheumatism, unspecified
- Q89.1 Malformations of the adrenal gland
- R57.8.0* Burn shock
- R57.9 Shock, unspecified
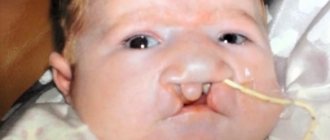
- S05.9 Injury to unspecified part of eye and orbit
- S06 Intracranial injury
- S09 Other and unspecified head injuries
- T78.2 Anaphylactic shock, unspecified
- T78.4 Allergy, unspecified
- T79.4 Traumatic shock
- T90.4 Consequences of injury to the eye, periorbital region
- Z100* CLASS XXII Surgical practice
- Z51.0 Radiotherapy course
- Z94.7 Presence of a transplanted cornea
Story
The pharmaceutical production enterprise KRKA-RUS LLC in Istra began operating in 2003. Then it was the first foreign investment in the Istra region, which amounted to more than 40 million euros.
In the first year of work, new equipment and all the necessary technological processes for the production of tablets, their packaging and packaging were mastered. Quality control laboratories and a microbiological laboratory were accredited, and all necessary licenses were obtained. The production of the first drugs began, production capacity was increased and the range was gradually expanded. From January 2011 to October 2013, construction of the second stage of the modern pharmaceutical plant KRKA-RUS II was carried out on the territory of KRKA-RUS LLC. The investment value amounted to 135 million euros - the largest investment of the Krka group abroad. As of 2021, the total investment in the development and construction of the plant amounted to 200 million euros . In the production of medicines, the most modern technological equipment and modern technologies are used, taking into account the requirements of the environment, human safety and rapid adaptation to market changes. In addition, at the end of 2013, a new high-rack automatic warehouse connected to the production building was put into operation. The warehouse's current capacity is up to 17,100 pallet spaces , which significantly expands the storage capabilities of manufactured products.
Currently, KRKA-RUS LLC produces 41 key drugs in 186 dosages and presentations. Work on registration and certification of products is also carried out here. Today, one of the most modern factories in Russia, the production capacity of the plant was 2.2 billion tablets in 2021. This figure is planned to increase to 3.0 billion tablets per year by 2022. The number of employees of the plant at the end of September 2021 was more than 490 people.
Directions for use and doses
IV, slow stream or drip (in acute and emergency conditions); i/m; local (into the pathological formation) administration is also possible.
The dosage regimen is individual and depends on the indications, the patient’s condition and his response to therapy.
To prepare a solution for intravenous drip infusion, you should use an isotonic sodium chloride solution or a 5% dextrose solution.
In the acute period for various diseases and at the beginning of therapy, Dexamethasone is used in higher doses. During the day, you can administer from 4 to 20 mg of Dexamethasone 3-4 times.
Doses of the drug for children (i.m.)
When carrying out replacement therapy (for adrenal insufficiency) - 0.0233 mg/kg or 0.67 mg/m2, divided into 3 doses, every 3rd day or 0.00776–0.01165 mg/kg or 0.233–0.335 mg/m2 daily. For other indications, the recommended dose is 0.02776 to 0.16665 mg/kg or 0.833 to 5 mg/m2 every 12 to 24 hours.
When the effect is achieved, the dose is reduced to maintenance or until treatment is stopped. The duration of parenteral use is usually 3-4 days, then switch to maintenance therapy with Dexamethasone tablets.
Long-term use of high doses of the drug requires a gradual dose reduction in order to prevent the development of acute adrenal insufficiency.
KRKA Awards in Russia
Decades of high-quality work, effective marketing and development in all directions have provided KRKA with a leading position in the Russian pharmaceutical market. The achievements of the KRKA company have been repeatedly awarded with honorary awards, certificates, and prizes.
Choice of Russian doctors
In 2021, at the opening ceremony of the Russian National Congress “Man and Medicine”, KRKA was awarded a special congress award “Choice of Russian Doctors”.
Product of the Year
In November 2021, KRKA was awarded the prestigious Product of the Year award for the high quality of veterinary products.
Influence Rating
According to the results of the sociological survey “Influence Rating”, conducted in 2014, the KRKA company is among the ten most influential Russian companies and among the ten most influential foreign pharmaceutical manufacturing companies*.
*Source: Farmvestnik No. 6/793 February 24, 2015
Order of Friendship
The Chairman of the Board and General Director of KRKA, Mr. Jože Colaric, for his great contribution to strengthening friendship and cooperation between the Russian Federation and the Republic of Slovenia in 2009, was awarded the Order of Friendship - one of the most honorable awards in the Russian Federation.
RBC 500 rating
KRKA PHARMA LLC ranks 470th in the 2021 ranking, which is 22 points higher than last year (492nd place).
This is a well-deserved position, and the positive dynamics of revenue growth indicates the stable development of the Russian division of the KRKA group of companies in the pharmaceutical market.
Platinum Ounce
The drug Enap was recognized as the best prescription drug for two years in a row (2006, 2007) according to the results of the Platinum Ounce competition. In April 2009, according to the results of the Platinum Ounce competition, the KRKA company was recognized as the “Best Foreign Manufacturer of 2008”, and the Director of the Regional Marketing Department, Elena Astakhova, was recognized as the “Best Functional Manager of 2008”. In 2011, the Director of the KRKA Group of Companies in the Russian Federation, Miran Bevec, became a laureate of the Platinum Ounce award in the category “Top Level Manager”. In 2012, the new brand KRKA Perineva was recognized as the most successful “Launch” of 2011 in the “Vector of the Year” category according to the results of the “Platinum Ounce” award.
Brand of the Year/EFFIE 2009 and 2012
National award “BRAND NO. 1 IN RUSSIA” In April 2010, the pharmaceutical company KRKA became the winner of the international professional award in the field of marketing communications “Brand of the Year / EFFIE 2009” in the category “Medical services. Pharmaceuticals." The KRKA company became the silver medalist of the award, receiving an honorary award for the effective marketing strategy of the Pikovit brand, one of the most popular brands on the children's vitamin market.
In 2012, the drug Nalgesin received the bronze award “Brand of the Year/EFFIE 2012” for an effective marketing strategy for promoting the drug Nalgesin.
Attractive employer 2015
In 2015, based on the results of a study conducted by the Superjob.ru portal among existing employer companies, KRKA received the status of “Attractive Employer - 2015”.
KRKA is a leader in cardiology and gastroenterology
In 2021, the KRKA company received an award as the best expert in the field of cardiology and gastroenterology at the XXIII Russian National Congress “Man and Medicine.
Golden pallet
The Golden Pallet was awarded to KRKA for achieving the best results in 2015 in terms of the following indicators:
- compliance with all logistics requirements (shipment “for factory boxes and pallets”, correct formation of cargo);
- organization of warehouse work for the assembly of goods at a high level;
- timely execution of electronic invoices, notification of shipment.
- prompt response to complaints.
Awards of KRKA-RUS LLC
Over the entire period of its existence, it has been awarded many certificates from the mayor of Istra, from the district and regional administration, including in 2008 the certificate “The best enterprise in the field of labor protection in the Istra region” was received. Head of the Information Technology Service S.A. Malorodov was recognized as the Absolute Winner of the All-Russian Competition “Manager of the Year - 2008” in the regional round of the competition in the Moscow Region and “Best Manager of a Structural Unit - 2009” in the All-Russian Competition “Manager of the Year - 2009”.
KRKA-RUS LLC is one of the most modern factories in Russia
Medicines from KRKA-RUS LLC meet the highest criteria for effectiveness and safety, and also have a price accessible to a wide range of patients. Most of the drugs produced by KRKA-RUS LLC are included in the list of vital and essential medicines published annually by the Ministry of Health and Social Development of the Russian Federation.
Today, one of the most modern factories in Russia, with a production capacity of more than 2 billion tablets and capsules per year. In the next five years, it is planned to increase capacity to 3 billion. The plant's activities are successfully inspected by the European Commissions for EU GMP; all products manufactured at the plant meet EU GMP requirements. 80% of the total need for medicines in the Russian Federation. In addition, there is an active process of registration of drugs in other CIS countries. Since 2011, export supplies of Russian-made drugs to the republics of Kazakhstan and Belarus have been established. Also, in September 2015, the first batches of manufactured products were shipped for sale in the European Union in Portugal. Since 2021, KRKA-RUS products are exported to Belarus, Kazakhstan, Uzbekistan, Ukraine, Azerbaijan, Georgia, Kyrgyzstan, Moldova, as well as to EU countries - Germany, Belgium, Ireland, Austria, Finland, Italy, France and Portugal
Awards
In 2010, for the achieved labor success, high professionalism and great contribution to the socio-economic development of the Moscow region, the team of KRKA-RUS LLC received diplomas of gratitude signed by the Governor of the Moscow Region.
In 2021, in the House of Government of the Moscow Region, Governor of the Moscow Region Vorobiev A.Yu. presented the sign of St. Sergei of Radonezh[1] to director Peter Zupan for his active participation in the development of foreign economic and investment cooperation in the Istra district of the Moscow Region. The award received indicates that the KRKA company, together with the Moscow region, has common development goals and follows the same mission.
In 2021, at the Government House of the Moscow Region, KRKA-RUS LLC was noted by the State Labor Inspectorate in the Moscow Region as employers guaranteeing compliance with the requirements of the labor legislation of the Russian Federation and was solemnly presented with a “Certificate of Trust in the Employer”. Receiving the certificate was the result of participation in the project “Declaration of the enterprise’s activities to implement the labor rights of employees and employers.”