Complivit Aqua D3
GENERAL INFORMATION
Registration number:
Trade name of the drug:
Complivit® Aqua D3
International nonproprietary name:
Colecalciferol
Dosage form:
drops for oral administration
Description:
Colorless, transparent or slightly opalescent liquid with a characteristic odor.
Pharmacotherapeutic group:
Calcium-phosphorus metabolism regulator.
ATX Code:
A11СС05
Compound
for 1 ml:
Active substance:
colecalciferol (vitamin D3) - 0.375* mg (15,000 IU)
Excipients:
macrogol glyceryl ricinoleate - 75,000 mg; sucrose (white sugar) - 250,000 mg; sodium hydrogen phosphate dodecahydrate - 7,000 mg; citric acid (citric acid monohydrate) - 0.430 mg; anethole - 0.825 mg; benzyl alcohol - 15,000 mg; purified water to 1 ml.
* 1 mg of Colecalciferol corresponds to 40,000 IU of vitamin D3 activity.
PHARMACOLOGICAL PROPERTIES
Pharmacodynamics
Vitamin D3 is an active antirachitic factor. The most important function of vitamin D3 is to regulate calcium and phosphate metabolism, which promotes proper mineralization and skeletal growth. Vitamin D3 is a natural form of vitamin D that is formed in humans in the skin under the influence of sunlight. Compared to vitamin D2, it is characterized by 25% higher activity. Colecalciferol plays a significant role in the absorption of calcium and phosphates from the intestine, in the transport of mineral salts and in the process of bone calcification, and also regulates the excretion of calcium and phosphates by the kidneys. The concentration of calcium ions in the blood determines the maintenance of muscle tone of skeletal muscles, myocardial function, promotes nervous stimulation, and regulates the process of blood clotting. Vitamin D is necessary for the normal function of the parathyroid glands and is also involved in the functioning of the immune system, influencing the production of lymphokines. Lack of vitamin D in food, impaired absorption, calcium deficiency, as well as insufficient exposure to sunlight leads to: in children during a period of intensive growth - to rickets, in adults - to osteomalacia, in pregnant women symptoms of tetany may occur, in newborns - a disorder processes of bone tissue calcification. An increased need for vitamin D occurs in women during menopause due to the development of hormonal disorders leading to osteoporosis.
Pharmacokinetics.
An aqueous solution of vitamin D3 is absorbed better than an oil solution. In premature babies, there is insufficient formation and flow of bile into the intestines, which interferes with the absorption of vitamins in the form of oil solutions. After oral administration, colecalciferol is absorbed in the small intestine. Metabolized in the liver and kidneys. The half-life of colecalciferol from the blood is several days and may be prolonged in cases of renal failure. The drug penetrates the placental barrier and into mother's milk. Vitamin D3 has the property of cumulation. It is excreted from the body by the kidneys in small quantities, most of it is excreted in bile.
INDICATIONS FOR USE
Prevention of vitamin D deficiency and diseases associated with its deficiency (rickets, osteomalacia).
Treatment of rickets.
Complex therapy of osteoporosis of various origins.
CONTRAINDICATIONS
Hypersensitivity to the components of the drug, especially benzyl alcohol. Sucrase/isomaltase deficiency, fructose intolerance, glucose-galactose malabsorption. Hypervitaminosis of vitamin D. Increased level of calcium in the blood (hypercalcemia), increased excretion of calcium in the urine (hypercalciuria), urolithiasis (formation of calcium oxalate stones), incl. history of renal osteodystrophy with hyperphosphatemia, pseudohypoparathyroidism. Sarcoidosis. Acute and chronic diseases of the liver and kidneys, renal failure. Active form of pulmonary tuberculosis. Children up to 4 weeks of age.
CAREFULLY
In patients in a state of immobilization. In patients taking thiazide diuretics, as well as in patients with cardiovascular diseases taking cardiac glycosides (see section "Interaction with other drugs"). During pregnancy and breastfeeding (see section “Use during pregnancy and breastfeeding”) In infants with a predisposition to early overgrowth of the fontanelles (when the small size of the anterior crown is established from birth). When taking additional amounts of vitamin D and calcium (for example, as part of other drugs), in case of impaired excretion of calcium and phosphate in the urine, during treatment with benzothiadiazine derivatives and in immobilized patients (risk of developing hypercalcemia and hypercalciuria) (see section Special instructions). In patients with the following concomitant diseases: atherosclerosis, heart failure, organic heart disease, granulomatosis, hyperphosphatemia, phosphate nephrolithiasis, gastrointestinal diseases, gastric and duodenal ulcers, hypothyroidism. If you have one or more of the listed diseases and conditions, you should consult your doctor before taking the drug.
USE IN PREGNANCY AND BREASTFEEDING
During pregnancy, the drug should not be used in doses exceeding those recommended for the prevention of vitamin D deficiency (see section “Method of administration and dosage”), due to the possibility of teratogenic effects in case of overdose. Vitamin D3 should be prescribed with caution in women who are breastfeeding, because a drug taken in high doses by the mother may cause overdose symptoms in the child. When using the drug during pregnancy and breastfeeding, it is necessary to take into account the intake of vitamin D from other sources; the daily dose of vitamin D should not exceed 600 IU.
METHOD OF APPLICATION AND DOSES
Orally. The drug Complivit® Aqua D3 is taken orally in a spoonful of liquid. 1 drop contains about 500 IU of vitamin D3.
Prevention of rickets:
- full-term newborns from 4 weeks of life - 1 drop (500 IU) per day; - premature babies from 4 weeks of life - 2 drops (1000 IU) per day during the first year of life, then 1 drop (500 IU) per day. The drug should be used during the first two years of a child’s life, during periods of reduced insolation (especially in winter).
Treatment of rickets:
In the absence of visible deformations of the skeletal system (mild rickets) - 2-3 drops (1000-1500 IU) per day, continue treatment for 30 days. In the presence of deformations of the skeletal system, characteristic of moderate and severe rickets - 4-8 drops (2000-4000 IU) per day, continue treatment for 30-45 days, the dose of the drug and duration of therapy depend on the severity of the changes and are determined by the doctor ( see Special instructions).
Prevention of vitamin D deficiency and diseases associated with its deficiency (osteomalacia):
1 drop (500 IU) per day during the entire period accompanied by a deficiency of vitamin D. The minimum duration of the course of prophylaxis is 1 month.
In complex treatment of osteoporosis:
1-2 drops (500-1000 IU) per day for 3 months. Repeated courses of therapy are possible on the recommendation of a doctor, depending on the results of assessing markers of bone metabolism and calcium metabolism (see Special instructions).
SIDE EFFECT
The frequency of adverse reactions has not been determined.
Metabolic and nutritional disorders: hypercalcemia and hypercalciuria.
Nervous system disorders: headache.
Cardiovascular system disorders: increased blood pressure, arrhythmias.
Disorders of the respiratory system, chest and mediastinal organs: exacerbation of the tuberculosis process in the lungs.
Gastrointestinal disorders: decreased appetite, constipation, flatulence, nausea, abdominal pain or diarrhea.
Skin and subcutaneous tissue disorders: hypersensitivity reactions such as itching, skin rash and urticaria.
Musculoskeletal and connective tissue disorders: arthralgia, myalgia.
Renal and urinary tract disorders: renal dysfunction, polyuria.
If side effects occur, you should consult a doctor.
OVERDOSE
Symptoms of acute overdose of vitamin D3:
early manifestations
(due to hypercalcemia) - constipation or diarrhea, dry oral mucosa, headache, thirst, pollakiuria, nocturia, polyuria, anorexia, metallic taste in the mouth, nausea, vomiting, general weakness and fatigue, hypercalcemia, hypercalciuria, dehydration;
late manifestations
- bone pain, cloudiness of urine (appearance of hyaline casts in the urine, proteinuria, leukocyturia), increased blood pressure, skin itching, photosensitivity of the eyes, conjunctival hyperemia, arrhythmia, drowsiness, myalgia, nausea, vomiting, pancreatitis, gastralgia, weight loss, rarely - psychosis (mental changes) and mood changes.
Symptoms of chronic overdose of vitamin D3
(when taken for several weeks or months for adults in doses of 20000-60000 IU / day, children - 2000-4000 IU / day): calcification of soft tissues, kidneys, lungs, blood vessels, arterial hypertension, renal and chronic heart failure (these effects most often occur when hyperphosphatemia is added to hypercalcemia), growth impairment in children (long-term use at a dose of 1800 IU/day).
Treatment:
If the above symptoms appear, you should stop using the drug and consult a doctor. A diet low in calcium (for several weeks), consumption of large amounts of fluid, forced diuresis using furosemide, electrolytes, as well as the administration of glucocorticosteroids and calcitonin are indicated. If the kidneys are functioning properly, calcium levels can be significantly reduced by infusion of isotonic sodium chloride solution (3-6 liters over 24 hours) with the addition of furosemide and, in some cases, also sodium edetate at a dose of 15 mg/kg/hour, while constantly monitoring calcium levels and electrocardiogram data. With oligoanuria, on the contrary, hemodialysis (dialysate without calcium) is necessary. A specific antidote is unknown.
To prevent overdose, in some cases it is recommended to monitor the concentration of calcium in the blood.
INTERACTION WITH OTHER MEDICINES
Antiepileptic drugs (especially phenytoin and phenobarbital, primidone), rifampicin, cholestyramine reduce the reabsorption of vitamin D3. Use simultaneously with thiazide diuretics increases the risk of developing hypercalcemia. In such cases, it is necessary to constantly monitor the concentration of calcium in the blood. With hypervitaminosis D3, it is possible to enhance the effect of cardiac glycosides and increase the risk of arrhythmia due to the development of hypercalcemia (monitoring the concentration of calcium in the blood, an electrocardiogram, as well as adjusting the dose of cardiac glycoside are advisable). Concomitant therapy with glucocorticosteroids may reduce the effectiveness of vitamin D3. Long-term use of antacids containing aluminum and magnesium in combination with vitamin D3 may contribute to an increase in the concentration of aluminum and magnesium in the blood and, as a result, to the toxic effect of aluminum on bone tissue and hypermagnesemia in patients with renal failure. Cholestyramine, colestipol and mineral oils reduce the absorption of fat-soluble vitamins in the gastrointestinal tract and require an increase in their dose. Concomitant use of benzodiazepines increases the risk of hypercalcemia. Drugs containing high concentrations of calcium and phosphorus increase the risk of developing hyperphosphatemia. When used simultaneously with sodium fluoride, the interval between doses should be at least 2 hours; with oral forms of tetracyclines - at least 3 hours. Concomitant use with other vitamin D analogues increases the risk of developing vitamin D hypervitaminosis. Ketoconazole can inhibit both the biosynthesis and catabolism of 1,25(OH)2-colecalciferol. Vitamin D is an antagonist of drugs used for hypercalcemia: calcitonin, etidronate, pamidronate, plicamycin, gallium nitrate. Isoniazid and rifampicin can reduce the effect of the drug due to an increase in the rate of biotransformation. Vitamin D3 does not interact with food.
SPECIAL INSTRUCTIONS
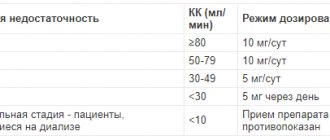
Avoid overdose. When taking the drug, the amount of vitamin D and calcium obtained from food and other medications should be taken into account. Too high doses of vitamin D3, used over a long period of time, or shock doses can cause chronic hypervitaminosis D3. High doses of calcium should not be taken at the same time as vitamin D3. The drug should be used with caution in patients with impaired urinary excretion of calcium and phosphate, during treatment with benzothiadiazine derivatives and in immobilized patients (risk of developing hypercalcemia and hypercalciuria). In such patients, calcium levels in the blood plasma and urine should be monitored. Vitamin D3 should not be taken if you have pseudohypoparathyroidism, as in this disease the need for vitamin D may be reduced, which may lead to a risk of long-term overdose. The main clinical manifestations of mild rickets include nervous excitability, anxiety, flinching at a sharp sound, a flash of light, sleep rhythm disturbances, superficial “anxious” sleep, sweating, itchy skin, baldness of the back of the head, pliability of the edges of the large fontanel. The presence of visible deformations of bone tissue is characteristic of moderate and severe rickets, which, as a rule, require hospitalization and complex therapy prescribed by a doctor based on the results of the examination. Repeated courses of therapy for osteoporosis are possible on the recommendation of a doctor, depending on the results of assessing markers of bone metabolism and calcium metabolism. If the doctor has prescribed a longer treatment than recommended in the instructions, then you should regularly (every three months of therapy) determine the level of calcium in the blood serum and urine, and also assess kidney function by measuring the level of creatinine in the blood serum. If necessary, the dose can be adjusted by the doctor according to the level of calcium in the blood serum. In case of hypercalcemia or signs of renal impairment, the dose of the drug should be reduced or treatment should be suspended. If the level of calcium in the urine exceeds 7.5 mmol/24 hours (300 mg/24 hours), it is recommended to reduce the dose of the drug or suspend treatment.
INFLUENCE ON THE ABILITY TO DRIVE VEHICLES AND MECHANISMS
There are no data on the possible effect of the drug on the ability to drive vehicles and machines.
RELEASE FORM
Drops for oral administration, 15000 IU/ml.
10 ml and 20 ml in dropper bottles made of dark (amber) glass, sealed with polyethylene dropper stoppers and plastic caps with first opening control.
One dropper bottle along with instructions for use is placed in a cardboard pack.
Storage conditions
In a place protected from light at a temperature not exceeding 25 ° C. Keep out of the reach of children.
BEST BEFORE DATE
2 years.
Do not use after the expiration date stated on the package.
Conditions for dispensing from pharmacies
Over the counter.
MANUFACTURER INFORMATION
Manufacturer's name and address:
OJSC Pharmstandard-Leksredstva,
305022, Russia, Kursk, st. 2nd Aggregatnaya, 1a/18, tel./fax: (4712) 34-03-13
www.pharmstd.ru
Marketing authorization holder/organization receiving consumer complaints:
OTCPharm JSC, Russia, 123317, Moscow, st. Testovskaya, 10
Tel.
Fax
www.otcpharm.ru
Complivit Calcium D3 Forte chewable tablets No. 30 mint
Compound
Active substances:
| calcium carbonate | 1.25 g, |
| which corresponds to the calcium content | 500 mg |
| colecalciferol (vit. D3)* | 0.01 mg (400 IU) |
* in the form of granulate containing colecalciferol - 0.27%, DL-α-tocopherol - 0.0275%, medium chain triglycerides - 10.7%, sucrose - 36%, acacia gum - 22%, corn starch - 27%, calcium phosphate (E341) - 0.5 %, water - up to 100%).
Excipients: lactose monohydrate (milk sugar) - 333.118 mg, povidone (medium molecular weight polyvinylpyrrolidone, povidone K17) - 68.24 mg, polysorbate 80 - 1.731 mg, potato starch - 18.969 mg, croscarmellose sodium - 50.282 mg, citric acid - 3.325 mg, aspartame (E951) - 5.95 mg, magnesium stearate - 15.75 mg, peppermint leaf oil - 2.625 mg.
Pharmacokinetics
Colecalciferol
Vitamin D3 is easily absorbed from the small intestine (about 80% of the dose taken). Colecalciferol and its metabolites circulate in the blood bound to a specific globulin. Colecalciferol is converted in the liver by hydroxylation to 25-hydroxycalciferol. It is then converted in the kidneys to the active form 1,25-hydroxycalciferol. 1,25-hydroxycalciferol is a metabolite responsible for increasing calcium absorption. Unmetabolized vitamin D3 is stored in adipose and muscle tissue. Excreted through the intestines and kidneys.
Calcium
Calcium is absorbed in ionized form from the proximal small intestine through an active, D-vitamin dependent transport mechanism. Typically, the amount of calcium absorbed from the gastrointestinal tract is approximately 30% of the dose taken. 99% of calcium in the body is concentrated in the hard tissues of teeth and bones. The remaining 1% is found in intra- and extracellular fluids. About 50% of the total calcium content in the blood is in physiological active ionized form, of which approximately 10% is complexed with citrate, phosphate or other anions, the remaining 40% is associated with proteins, primarily albumin. Calcium is excreted by the intestines, kidneys and sweat glands. Renal excretion depends on glomerular filtration and tubular reabsorption of calcium.
Indications for use
- Prevention and complex therapy of osteoporosis (menopausal, senile, “steroidal”, idiopathic) and its complications (bone fractures).
- Replenishment of calcium and/or vitamin D3 deficiency.
Contraindications
Hypersensitivity to the components of the drug (including lactose intolerance, lactase deficiency, sucrase/isomaltase deficiency, fructose intolerance, glucose-galactose malabsorption), hypercalcemia, hypercalciuria, calcium nephrourolithiasis, hypervitaminosis D, decalcifying tumors (myeloma, bone metastases, sarcoidosis ), osteoporosis due to immobilization, phenylketonuria (contains aspartame), pulmonary tuberculosis (active form), chronic renal failure, children under 3 years of age.
With caution: pregnancy, lactation period.
Directions for use and doses
Inside, chew or swallow whole, preferably during meals, with water.
Adults: for the treatment of osteoporosis - 1 tablet 2-3 times a day, for the prevention of osteoporosis - 1 tablet 2 times a day.
To compensate for calcium and/or vitamin D3 deficiency:
- Adults and children over 12 years old - 1 tablet 1-2 times a day.
- Children from 3 to 12 years old - 1 tablet per day or as prescribed by a doctor.
Storage conditions
At a temperature not higher than 25 °C. Keep out of the reach of children.
Best before date
3 years. Do not use after the expiration date stated on the package.
special instructions
To avoid overdose, additional vitamin D3 intake from other sources must be taken into account.
Complivit® Calcium Dz Forte contains aspartame, which is transformed into phenylalanine in the body, so the drug should not be taken by patients suffering from phenylketonuria.
During the treatment period, it is necessary to constantly monitor the excretion of calcium in the urine and the concentration of calcium and creatinine in the blood plasma (in case of calciuria exceeding 7.5 mmol/day (300 mg/day), it is necessary to reduce the dose or stop taking it).
In older people, the need for calcium is 1500 mg/day, for vitamin D3 - 0.5-1 thousand IU/day.
Description
Calcium-phosphorus metabolism regulator.
Pharmacodynamics
A combined drug whose effect is determined by its constituent components.
Regulates the exchange of calcium and phosphates, reduces resorption and increases bone density, replenishes the lack of calcium and vitamin D3 in the body, enhances the absorption of calcium in the intestines and the reabsorption of phosphates in the kidneys, promotes bone mineralization. Calcium is involved in the formation of bone tissue, blood clotting, maintaining stable cardiac activity, and in carrying out the processes of transmission of nerve impulses.
Vitamin D3 (colecalciferol) - promotes the absorption of calcium in the intestines, the formation and mineralization of bone and dental tissue. The use of calcium and vitamin D3 prevents the increase in the production of parathyroid hormone, which is a stimulator of increased bone resorption.
Side effects
Allergic reactions. Dyspeptic disorders of the gastrointestinal tract (constipation, flatulence, nausea, stomach pain, diarrhea). Hypercalcemia and hypercalciuria (increased calcium levels in the blood and urine).
Use during pregnancy and breastfeeding
The daily dose should not exceed 1500 mg of calcium and 600 IU of vitamin D3. Hypercalcemia, which develops against the background of an overdose, during pregnancy can cause defects in the mental and physical development of the child. Vitamin D and its metabolites can pass into breast milk, so it is necessary to consider the intake of calcium and vitamin D from other sources in the mother and child.
Interaction
The activity of vitamin D3 may decrease when used concomitantly with phenytoin or barbiturates.
During simultaneous treatment with cardiac glycosides, monitoring of the ECG and clinical condition is necessary, since calcium preparations can potentiate the therapeutic and toxic effects of cardiac glycosides.
Calcium and vitamin D3 supplements may increase the absorption of tetracyclines: from the gastrointestinal tract. Therefore, the time interval between doses of drugs should be at least three hours.
To prevent decreased absorption of bisphosphonate drugs or sodium fluoride, it is recommended to take Complivit Calcium Dz Forte no earlier than two hours after taking them.
Glucocorticosteroids reduce the absorption of calcium, so treatment with glucocorticosteroids may require an increase in the dose of Complivit Calcium Dz Forte.
Simultaneous treatment with cholestyramine or laxatives based on mineral or vegetable oil may reduce the absorption of vitamin D3.
With simultaneous use of thiazide diuretics, the risk of hypercalcemia increases, as they increase tubular reabsorption of calcium. Furosemide and other “loop” diuretics, on the contrary, increase calcium excretion by the kidneys.
Overdose
If you notice signs of overdose, consult a doctor.
Symptoms: thirst, polyuria, loss of appetite, nausea, vomiting, constipation, dizziness, weakness, headache, fainting, coma; with long-term use: calcification of blood vessels and tissues.
Treatment: introduction of large amounts of fluid into the body, use of loop diuretics (for example, furossmide), glucocorticosteroids, calcitonin, bisphosphoiates. If clinical symptoms of overdose develop, the concentration of calcium and creatinine in the blood should be determined. In case of increased concentration of calcium or creatinine in the blood serum, the dose of the drug should be reduced or treatment should be temporarily stopped.