Xyzal®
The pharmacokinetic parameters of levocetirizine change linearly and practically do not differ from the pharmacokinetics of cetirizine.
Suction
After oral administration, the drug is quickly and completely absorbed from the gastrointestinal tract. Eating does not affect the completeness of absorption, although its speed decreases. In adults, after a single dose of the drug in a therapeutic dose (5 mg), the maximum concentration (Cmax) in the blood plasma is reached after 0.9 hours and is 270 ng/ml, after repeated administration at a dose of 5 mg - 308 ng/ml. Equilibrium concentration is achieved after 2 days.
Distribution
Levocetirizine is 90% bound to plasma proteins. The volume of distribution (Vd) is 0.4 l/kg. Bioavailability reaches 100%.
Metabolism
In small quantities (<14%) it is metabolized in the body by N- and O-dealkylation (unlike other H1-histamine receptor antagonists, which are metabolized in the liver using the cytochrome system) to form a pharmacologically inactive metabolite. Due to negligible metabolism and lack of metabolic potential, interaction of levocetirizine with other drugs is unlikely.
Removal
In adults, the half-life (T1/2) is 7.9 ± 1.9 hours; in young children T1/2 is shortened. In adults, the total clearance is 0.63 ml/min/kg. About 85.4% of the administered dose of the drug is excreted unchanged by the kidneys through glomerular filtration and tubular secretion; about 12.9% - through the intestines.
Selected patient groups
Patients with kidney failure
In patients with renal failure (creatinine clearance (CC) < 40 ml/min), the clearance of the drug is reduced and T1/2 is prolonged (for example, in patients on hemodialysis, the total clearance is reduced by 80%), which requires a corresponding change in the dosage regimen . Less than 10% of levocetirizine is removed during a standard 4-hour hemodialysis procedure.
Patients with liver failure
The pharmacokinetics of levocetirizine in patients with hepatic impairment have not been studied. In patients with chronic liver diseases (hepatocellular, cholestatic and biliary cirrhosis) receiving the racemic compound cetirizine at a dose of 10 or 20 mg as a single dose, an increase in half-life by 50% and a decrease in clearance of the drug by 40% were observed, compared with healthy people.
Children
Data from a study of the pharmacokinetics of the drug in 14 children aged 6 to 11 years weighing from 20 to 40 kg with a single oral dose of 5 mg of levocetirizine showed that Cmax and area under the curve (AUC) were approximately twice as high as in healthy adults with cross-control. The mean Cmax was 450 ng/ml, the maximum concentration was reached after an average of 1.2 hours, the total body weight-adjusted clearance was 30% higher, and the half-life was 24% shorter in children than in adults. Specific pharmacokinetic studies have not been conducted in children under 6 years of age. A retrospective pharmacokinetic analysis was conducted in 323 patients (181 children aged 1 to 5 years, 18 children aged 6 to 11 years, and 124 adults aged 18 to 55 years) who received one or more doses of levocetirizine 1.25 mg. up to 30 mg. Data obtained during the analysis showed that taking the drug at a dose of 1.25 mg in children aged 6 months to 5 years leads to plasma concentrations similar to those in adults when taking 5 mg of the drug once a day.
Elderly patients
Pharmacokinetic data in elderly patients is limited. When repeated dosing of levocetirizine 30 mg once daily for 6 days in 9 elderly patients (ages 65 to 74 years) total clearance was approximately 33% lower than that in younger adults. The distribution of cetirizine racemate has been shown to be more dependent on renal function than on age. This statement may also apply to levocetirizine, since both levocetirizine and cetirizine are excreted primarily in the urine. Therefore, in elderly patients, the dose of levocetirizine should be adjusted depending on renal function.
Xyzal (drops), 1 piece, 10 ml, 5 mg/ml, drops for oral administration
Inside,
during meals or on an empty stomach. Swallow film-coated tablets whole with a small amount of water. To take the drug in the form of drops, use a teaspoon. If necessary, the dose of the drug can be diluted in a small amount of water immediately before use.
Adults and children over 6 years of age:
The daily dose is 5 mg (1 tablet or 20 drops) once.
Children from 2 to 6 years old:
1.25 mg (5 drops) 2 times a day; daily dose - 2.5 mg (10 drops).
Since levocetirizine is excreted from the body by the kidneys, when using the drug in patients with renal failure and elderly patients, the dose should be adjusted depending on the creatinine clearance. Creatinine clearance for men can be calculated from serum creatinine concentration using the following formula:
Creatinine Cl, ml/min
Creatinine clearance for women can be calculated by multiplying the resulting value by a factor of 0.85.
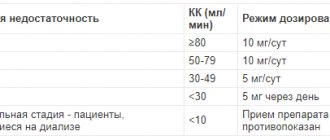
Elderly patients with normal renal function do not require dose reduction; in patients with chronic renal failure, dose calculation should be carried out taking into account creatinine clearance according to the table below.
| Kidney failure | Creatinine Cl, ml/min | Dose and frequency of administration |
| Absent (normal) | ≥80 | 5 mg/day |
| Lightweight | 50–79 | 5 mg/day |
| Average | 30–49 | 5 mg once every 2 days |
| Heavy | <30 | 5 mg once every 3 days |
| End stage - patients on hemodialysis | <10 | taking the drug is contraindicated |
For patients with renal and hepatic insufficiency, dosing is carried out according to the table above.
In patients with impaired liver function only, no dosage adjustment is required.
Duration of taking the drug:
when treating seasonal (intermittent) rhinitis (the presence of symptoms less than 4 days a week or their total duration less than 4 weeks), the duration of treatment depends on the nature of the disease; Treatment may be stopped when symptoms disappear and resumed when symptoms appear. When treating year-round (persistent) allergic rhinitis (the presence of symptoms more than 4 days a week and their total duration of more than 4 weeks), treatment is possible throughout the entire period of exposure to allergens. There is clinical experience with the continuous use of Xyzal® tablets in adult patients for up to 6 months.
Xizal
The histamine H1 receptor blocker, an enantiomer of cetirizine, belongs to the group of competitive histamine antagonists. The affinity for histamine H1 receptors of levocetirizine is 2 times higher than that of cetirizine.
Levocetirizine has an effect on the histamine-dependent stage of allergic reactions, and also reduces the migration of eosinophils, reduces vascular permeability, and limits the release of inflammatory mediators. Prevents the development and facilitates the course of allergic reactions, has an antiexudative, antipruritic effect, and has virtually no anticholinergic and antiserotonin effects. In therapeutic doses it has virtually no sedative effect.
Pharmacokinetics
Suction
The pharmacokinetic parameters of levocetirizine change linearly and practically do not differ from the pharmacokinetics of cetirizine. After oral administration, levocetirzine is rapidly absorbed from the gastrointestinal tract. Food intake does not affect the degree of absorption, although its rate decreases. After a single oral administration at a therapeutic dose, Cmax in blood plasma in adults is reached after 0.9 hours and is 207 ng/ml, after repeated administration at a dose of 5 mg/day - 308 ng/ml. Bioavailability is 100%.
Distribution
Css is achieved after 2 days. The binding of levocetirizine to plasma proteins is 90%. Vd is 0.4 l/kg.
Metabolism
Less than 14% is metabolized in the liver by N- and O-dealkylation (unlike other histamine H1 receptor blockers, which are metabolized in the liver with the participation of cytochrome P450 isoenzymes) to form a pharmacologically inactive metabolite. Due to the low rate of metabolism and lack of metabolic potential, interaction of levocetirizine with other drugs is unlikely.
Removal
In adults it is 7.9±1.9 hours, total clearance is 0.63 ml/min/kg. About 85.4% of the dose is excreted unchanged by the kidneys by glomerular filtration and tubular secretion; about 12.9% is excreted through the intestines.
Pharmacokinetics in special clinical situations
In patients with renal failure (creatinine clearance less than 40 ml/min), the clearance of levocetirizine decreases and T1/2 increases (in patients on hemodialysis, the total clearance decreases by 80%), which requires a corresponding change in the dosage regimen. Less than 10% of levocetirizine is removed during a standard 4-hour hemodialysis procedure.
In young children, T1/2 is shortened.
Xyzal drops for oral administration 5 mg/ml 10 ml bottle 1 pc. in Moscow
Pharmacological action: Pharmacodynamics
Levocetirizine, the R-enantiomer of cetirizine, is a competitive histamine antagonist. Blocks H1-histamine receptors. The affinity for H1-histamine receptors of levocetirizine is 2 times higher than that of cetirizine.
Levocetirizine has an effect on the histamine-dependent stage of allergic reactions, and also reduces the migration of eosinophils, vascular permeability, and limits the release of inflammatory mediators.
Levocetirizine prevents the development and alleviates the course of allergic reactions, has an antiexudative, antipruritic effect and practically does not cause anticholinergic and antiserotonin effects. In therapeutic doses it has virtually no sedative effect.
Pharmacokinetics
The pharmacokinetic parameters of levocetirizine change linearly and practically do not differ from the pharmacokinetics of cetirizine.
Suction.
After oral administration, levocetirizine is quickly and completely absorbed from the gastrointestinal tract. Eating does not affect the completeness of absorption, although its speed decreases. In adults, after a single dose of a therapeutic dose (5 mg), Cmax in blood plasma is 270 ng/ml and is reached after 0.9 hours, after repeated administration of a dose of 5 mg - 308 ng/ml. Css is achieved after 2 days.
Distribution.
Levocetirizine is 90% bound to plasma proteins. Vd is 0.4 l/kg. Bioavailability reaches 100%.
Metabolism.
In small quantities (<14%) it is metabolized in the body by N- and O-dealkylation (unlike other H1-histamine receptor antagonists, which are metabolized in the liver using the cytochrome system) to form a pharmacologically inactive metabolite. Due to negligible metabolism and lack of metabolic potential, interaction of levocetirizine with other drugs seems unlikely.
Excretion.
In adults, T1/2 is (7.9±1.9) hours; in young children T1/2 is shortened. In adults, the total clearance is 0.63 ml/min/kg. About 85.4% of the dose taken is excreted unchanged by the kidneys through CP and tubular secretion; about 12.9% - through the intestines.
Selected patient groups
Kidney failure.
In patients with renal failure (Cl creatinine <40 ml/min), the clearance of levocetirizine is reduced and T1/2 is prolonged (for example, in patients on hemodialysis, the total clearance is reduced by 80%), which requires a corresponding change in the dosage regimen. Less than 10% of levocetirizine is removed during a standard 4-hour hemodialysis procedure.
Children.
Data from a study of the pharmacokinetics of levocetirizine in 14 children aged 6 to 11 years weighing from 20 to 40 kg with a single oral dose of 5 mg showed that the Cmax and AUC values are approximately 2 times higher than those in healthy adults. The average Cmax value was 450 ng/ml, Tmax averaged 1.2 hours, total clearance taking into account body weight was 30% higher, and T1/2 was 24% shorter in children than the corresponding values in adults. A retrospective pharmacokinetic analysis was conducted in 323 patients (181 children aged 1 to 5 years, 18 children aged 6 to 11 years, and 124 adults aged 18 to 55 years) who received one or more doses of levocetirizine 1.25 mg to 30 mg. Data obtained during the analysis showed that taking the drug at a dose of 1.25 mg in children aged 6 months to 5 years leads to plasma concentrations corresponding to those in adults when taking 5 mg of the drug once a day.
Elderly patients.
Pharmacokinetic data in elderly patients is limited. When repeated dosing of levocetirizine 30 mg once daily for 6 days in 9 elderly patients (age 65 to 74 years) total clearance was approximately 33% lower than that in younger adults. The distribution of cetirizine racemate has been shown to be more dependent on renal function than on age. This statement may also apply to levocetirizine, because Both drugs, levocetirizine and cetirizine, are excreted primarily in the urine. Therefore, in elderly patients, the dose of levocetirizine should be adjusted depending on renal function.