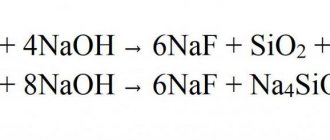Story
The history of nitroglycerin begins with the Italian chemist Ascaño Sobrero. He first synthesized this substance in 1846. It was originally given the name pyroglycerin. Already Sobrero discovered its great instability - nitroglycerin could explode even from weak shocks or impacts.
The explosive power of nitroglycerin theoretically made it a promising reagent in the mining and construction industries - it was much more effective than the types of explosives that existed at that time. However, the mentioned instability created too great a threat during its storage and transportation - so nitroglycerin was shelved.
Things moved a little forward with the appearance of Alfred Nobel and his family - father and sons established industrial production of this substance in 1862, despite all the dangers associated with it. However, what had to happen sooner or later happened - there was an explosion at the factory, and Nobel’s younger brother died. After suffering grief, his father retired, but Alfred managed to continue production. To increase safety, he mixed nitroglycerin with methanol - the mixture was more stable, but very fire hazardous. It still wasn't a final decision.
It was dynamite - nitroglycerin absorbed by kieselguhr (sedimentary rock). The explosiveness of the substance has decreased by several orders of magnitude. Later, the mixture was improved, kieselguhr was replaced with more effective stabilizers, but the essence remained the same - the liquid was absorbed and stopped exploding at the slightest shock.
Inventor of dynamite - Nobel. History of the invention of dynamite
Alfred Bernhard Nobel was a Swedish chemist, engineer and industrialist who invented dynamite and more powerful explosives, and founded the Nobel Prize.
Biography
The future inventor of dynamite, Alfred Nobel, was born in Stockholm (Sweden) on October 21, 1833. He was the fourth son of Emmanuel and Caroline Nobel. Emmanuel was an engineer who married Caroline Andriette Alsel in 1827. The couple had eight children, of whom only Alfred and three brothers reached adulthood. As a child, Nobel was often ill, but from an early age he showed a keen curiosity. He was interested in explosives and learned basic engineering from his father. The father, meanwhile, suffered failures in various business ventures until he moved to St. Petersburg in 1837, where he became a successful manufacturer of mines and tools.
Life abroad
In 1842, Nobel's family left Stockholm to join their father in St. Petersburg. Alfred's now wealthy parents could now hire him private teachers, and he proved to be an eager student. By the age of 16, Nobel had become a competent chemist, fluent in English, German, French and Russian.
In 1850, Alfred left Russia to spend a year in Paris studying chemistry and then four years in the United States working under John Erickson, who was building the battleship Monitor. Upon returning to St. Petersburg, he worked in his father's factory, which produced military equipment during the Crimean War. After the end of hostilities in 1856, the company had difficulty transitioning to manufacturing equipment for steamships and went bankrupt in 1859.
Bet on nitroglycerin
The future inventor of dynamite did not stay in Russia and returned to Sweden with his parents, and his brothers Robert and Ludwig decided to save the remains of the family enterprise. Alfred soon began experimenting with explosives in a small laboratory on his father's estate. At that time, the only reliable explosive used in mines was black powder. The newly created liquid nitroglycerin was much more powerful, but it was so unstable that it could not provide any safety. However, in 1862 Nobel built a small plant to produce it, while conducting research in the hope of finding a way to control its detonation.
In 1863 he invented a practical detonator consisting of a wooden plug inserted into a large charge of nitroglycerin stored in a metal container. The explosion of a small charge of black powder in the plug detonated a much more powerful charge of liquid explosive. This detonator marked the beginning of Nobel's reputation as an inventor, as well as the fortune he would make as an explosives manufacturer.
In 1865, Alfred created an improved detonator cap, which consisted of a small metal cap containing a charge of mercury fulminate, detonated either by impact or moderate heat. This invention marked the beginning of the modern use of explosives.
Accident
Nitroglycerin itself, however, was difficult to transport and extremely dangerous to handle. So dangerous that Nobel's plant exploded in 1864, killing his younger brother Emil and others. Undeterred by this tragic accident, Alfred built several factories to produce nitroglycerin for use with his primers. These plants were as safe as the knowledge of the time allowed, but accidental explosions continued to occur.
Lucky Accident
Nobel's second important invention was dynamite. In 1867, he accidentally discovered that nitroglycerin was completely absorbed by porous silica, and the resulting mixture was much safer to use and easier to handle. Alfred, the inventor of dynamite (from the Greek δύναμις, “power”), received patents for it in Great Britain (1867) and the USA (1868). The explosive made its creator famous all over the world, and soon it began to be used in the construction of tunnels and canals, and the construction of railways and roads.
Rattlesnake jelly
In the 1870s and 80s, dynamite inventor Alfred Nobel built a network of explosives factories across Europe and formed a network of corporations to sell them. He also continued to experiment to find the best ones, and in 1875 he created a more powerful form of dynamite, jelly fulminate, which he patented the following year. Again by accident, he discovered that a mixture of a solution of nitroglycerin with a loose fibrous substance known as nitrocellulose formed a dense, plastic material that was highly resistant to water and had greater explosive power. In 1887, Nobel introduced ballistite, a nitroglycerin smokeless powder and precursor to cordite. Although Alfred held patents for dynamite and other explosives, he was in constant conflict with competitors who stole his technology, which forced him into protracted patent disputes on several occasions.
Oil, weapons, wealth
Nobel's brothers, Ludwig and Robert, meanwhile developed newly discovered oil fields near Baku (now in Azerbaijan) on the Caspian Sea and became very rich men themselves. Sales of explosives around the world, as well as participation in the brothers' companies in Russia, brought Alfred a huge fortune. In 1893, the inventor of dynamite became interested in the Swedish arms industry, and the following year he bought an iron smelter in Bofors, near Värmland, which became the center of a famous arms factory. Besides explosives, Nobel invented many other things such as rayon and leather, and in total he registered more than 350 patents in various countries.
Ascetic, writer, pacifist
The inventor of dynamite, Nobel, was a complex personality, which puzzled his contemporaries. Although his business interests required him to travel almost constantly, he remained a solitary recluse who was prone to bouts of depression. Alfred led a solitary and simple life, he was a man of ascetic habits, but he could also be a polite host, a good listener, and a man of insightful mind.
The inventor of dynamite never married, and apparently preferred the joy of creativity to romantic attachments. He had an abiding interest in literature, writing plays, novels and poetry that remained almost entirely unpublished. He had amazing energy and found it difficult to relax after intense work. Among his contemporaries he enjoyed a reputation as a liberal or even a socialist, but in fact he distrusted democracy, was against women's suffrage and supported a soft paternalism towards his many employees. Although the Swedish inventor of dynamite was essentially a pacifist and hoped that the destructive power of his creations would help end war, his view of humanity and nations was pessimistic.
Surprise will
By 1895, Alfred developed angina, and on December 10 of the following year he died of a cerebral hemorrhage at his villa in San Remo (Italy). By this time, Nobel's business empire consisted of more than 90 factories producing explosives and ammunition. His will, drawn up in Paris on November 27, 1895 and deposited in a bank in Stockholm, contained a big surprise for his family, friends and the general public. The inventor of dynamite was always generous to humanitarian and scientific charities and left much of his fortune in trust to establish the most highly regarded international award, the Nobel Prize.
Death of a Death Dealer
One can only guess about the reasons for this decision. He was secretive and did not tell anyone about any of his decisions in the months leading up to his death. The most plausible possibility is that a strange incident in 1888 may have set off a chain of thought that led to his will. That same year, Alfred's brother Ludwig died while in Cannes, France. The French press reported his brother's death, but confused him with Alfred, and one of the newspapers published the headline "The Merchant of Death Died." Perhaps the inventor of dynamite instituted the prizes to avoid precisely the kind of posthumous reputation expressed by this premature obituary. It is obvious that the awards established reflect his interest in the fields of chemistry, physics, physiology and literature. There is also ample evidence that his friendship with the prominent Austrian pacifist Bertha von Suttner inspired him to create the Peace Prize.
Nobel himself, however, remains a figure of paradox and contradiction: a brilliant solitary man, part pessimist and part idealist, who invented the powerful explosives used in modern warfare and established the world's most prestigious prizes for intellectual services rendered to humanity.
fb.ru
Physical and chemical properties
Nitroglycerin is a nitroester of nitric acid and glycerin. Under normal conditions it is a yellowish, viscous oily liquid. Nitroglycerin is insoluble in water. Nobel took advantage of this property: in order to prepare nitroglycerin for use after transportation and free it from methanol, he washed the mixture with water - methyl alcohol dissolved in it and left, but the nitroglycerin remained. The same property is used in the production of nitroglycerin: the synthesis product is washed with water to remove any remaining reagents.
Nitroglycerin hydrolyzes (to form glycerol and nitric acid) when heated. Alkaline hydrolysis occurs without heating.
Stabilization of nitroglycerin. Dynamite
Nobel's first experiment in stabilizing nitroglycerin was dynamite - kieselguhr completely absorbed the liquid, and the mixture was safe (until, of course, it was activated in a demolition bomb). The reason why diatomaceous earth is used is the capillary effect. The presence of microtubules in this rock determines the effective absorption of liquid (nitroglycerin) and its retention there for a long time.
Obtained in the laboratory
The reaction for producing nitroglycerin in the laboratory is now still the same as that used by Sobrero - esterification in the presence of sulfuric acid. First, a mixture of nitric and sulfuric acids is taken. Acids need to be concentrated, with a small amount of water. Next, glycerin is gradually added to the mixture in small portions with constant stirring. The temperature must be kept low, since in a hot solution, instead of esterification (ester formation), oxidation of glycerol with nitric acid will occur.
But since the reaction releases a large amount of heat, the mixture must be constantly cooled (usually this is done with ice). As a rule, it stays around 0 ° C; exceeding 25 ° C can lead to an explosion. Temperature is monitored continuously using a thermometer.
Nitroglycerin is heavier than water, but lighter than mineral (nitric and sulfuric) acids. Therefore, in the reaction mixture the product will lie in a separate layer on the surface. After the reaction is completed, the vessel must be cooled further, wait until the maximum amount of nitroglycerin has accumulated in the top layer, and then pour it into another container with cold water. Then comes intensive washing in large volumes of water. This is necessary in order to clean nitroglycerin as best as possible from all impurities. This is important, because when combined with the remains of unreacted acids, the explosive hazard of the substance increases several times.
Nitroglycerine*
— Glycerol C3H5 (HO) 3, under the action of nitric acid or a mixture of nitric and sulfuric acids, can form nitric acid esters: C 3H5 (HO) 2(NO3), C 3H5 (HO)(NO 3)2 and C 3H5 ( NO 3)3.
Of these, only two are currently known - mononitrogen and trinitrogen. The first, obtained by mixing glycerin with moderately diluted nitric acid (1 part HNO 3 to 3 parts H 2 O), is a liquid that is easily soluble in water and alcohol, almost insoluble in ether and does not explode on impact. Trinitrogen ether is obtained by the action of a mixture of the strongest acids, nitric and sulfuric, on glycerin and differs from the previous one in its relation to solvents, and especially in its extremely strong explosiveness upon rapid heating and impact. This last ether is the powerful explosive substance that was first prepared by Sobrero in the Pelus laboratory in 1847 and has since been called N in explosive technology. Among the first persons who gave impetus to its use in practice, Professor Zinin should be named ( 1854) and artillery lieutenant (later lieutenant general) V.F. Petrushevsky, but the main merit in this regard undoubtedly belongs to the Swedish engineer. Alfred Nobel, who invented a way (by turning it into dynamite) to make it safe enough for transportation and handling. Currently, N. production represents one of the prominent branches of the manufacturing industry.
1) To prepare nitroglycerin, the general reaction for producing nitric acid esters of alcoholic substances is used, i.e. the action on glycerin (1 part) with strong nitric acid (3 parts) in the presence of concentrated sulfuric acid (6 parts):
C 3H5 (HO) 3 + 3HNO3 = C 3H5 (NO 3)3 + 3H2O.
The presence of sulfuric acid is necessary, on the one hand, to absorb the water released during the reaction, which, otherwise, diluting the nitric acid, thereby preventing the completeness of nitration (incomplete nitrogen esters of glycerol would begin to be obtained), on the other hand, to release the resulting N. from a solution in nitric acid, since it, being highly soluble in this acid, does not dissolve in a mixture of it with sulfuric acid. This reaction of N. formation is accompanied by significant self-heating, because both the esterification of glycerol with nitric acid and the combination of the resulting water with sulfuric acid separate heat. If, as a result of self-heating, the temperature of the mixture increased to 50°, then the action of the acids would easily be directed in the other direction: the oxidation of glycerin and nitrogen would begin, accompanied by the rapid release of nitrogen oxides (red-brown vapors) and even greater self-heating, which could finally lead to to the explosion of the resulting nitroglycerin. Therefore, the reaction should be carried out with constant cooling of the mixture of acids and add glycerin to the latter little by little, stirring each poured portion. Formed directly upon contact with N. acids, having a lower beat. weighing (1.6) compared to the acid mixture (not less than 1.7), floats to the surface, from where it can be collected after the reaction is completed. But self-heating during the preparation of N. can be counteracted in another way, namely by forcing part of the heat to be released before the formation of N. itself and, in particular, by slowing down the reaction of this formation by a preliminary change in the chemical state of the reacting substances. Boutmi and Faucher achieved this by first preparing two separate mixtures - sulfur-glycerin and sulfur-nitrogen. The latter is composed of equal parts of H 2 SO 4 n HNO 3, and the first of one part of C 3H5 (HO) 3 with a triple amount of H 2SO4, which produces sulfur-glycerol ether C 3H5 (HO) 2HSO4 with significant heat release. If both mixtures, after cooling, are mixed together in such a proportion that the ratio of the amounts of C 3H5 (HO) 3, H 2 SO 4 and HNO 3 is approximately the same as in the previous method, then: a) conversion of sulfur-glycerol ether in N. it proceeds gradually and slowly, so that it ends only after 12 or even 24 hours; b) the amount of heat released during this entire process is significantly reduced, since that part of the heat that occurs as a result of the combination of water with sulfuric acid has already been released earlier during the formation of the sulfur-glycerin mixture, and on the other hand, the glycerin itself, having turned into sulfur-glycerol ether, then lost part of its energy in the form of heat. Due to both of these reasons, the preparation of N. using this method cannot be accompanied by significant heating, since the reduced amount of heat during the slow course of the reaction has time to be transferred to the environment. The reason why the three-body system C 3H5 (HO) 3, H2SO4 and HNO 3, regardless of the initial state, is transformed so that glycerol preferentially combines with nitric acid, and sulfuric acid with the liberated water, according to Berthelot’s interpretation, is that that it is precisely such a final system of bodies that corresponds to the greatest separation of heat, i.e., the principle of maximum work accepted by this scientist as a general rule for the course of chemical reactions. In reality, the question is more complicated, because in the reaction of nitrogen formation, as in the vast majority of other reactions, the usual operation of the general law of chemical masses is observed. In fact, experiments show that from a given amount of glycerin, under ordinary preparation conditions, the theoretical yield of its trinitrogen ester is never obtained, namely: from 100 parts of C 3H5 (HO) 3 no more than 234, and usually about 210 parts. instead of 247. This is explained by the fact that, as a given amount of glycerol is added, less and less HNO 3 remains in the acid mixture, while the mass of H 2SO4 remains the same, until such a ratio finally occurs between the amounts of that and another acid, in which, in the presence of previously isolated water, glycerol is converted into incomplete nitrogen esters or even completely ceases to be nitrated, forming only sulfuric acid ester.
Factory preparation of N. can be done using both of these methods. But the Boutmi and Faucher method, used soon after its appearance at several factories (French - in Vonge; Belgian - in Namur; English - in Pamberey), is now completely abandoned, as less profitable (product yield is only 190 parts out of 100 including glycerin) and, moreover, (judging by the accidents at these factories) no safer compared to the usual method, especially since with such prolonged contact of N. with acids (12 - 24 hours), which occurs during nitration by This method still does not prevent the possibility of developing an oxidation reaction with the rapid release of nitrogen oxides. As for the usual method, being generally more economically advantageous, it is, at the same time, now considered safer if the starting materials are of proper purity. The form in which it is used in factories may vary greatly in its details. At first, N. was usually prepared in small portions, similar to how it is done in laboratories. So, according to Kopp, to 2 8 00 g of an acid mixture in a clay or cast iron pot placed in a vessel with water (5 - 6 liters), add 350 g of glycerin from a mug with constant stirring with a glass or iron rod; the resulting mixture is poured into a separating funnel and the settled acids are released, and N. is poured into the above-mentioned vessel with water for washing. Later, on the one hand, they began to increase the portions of glycerin processed at a time, and on the other, to use various mechanical devices for stirring during nitration. An important improvement in the latter respect was the use (for the first time at the Mowbray factories in Massachusetts) of blowing the mixture with compressed air, which produces not only agitation, but also cooling due to its expansion: clay pots placed in a common water bath around an exhaust pipe are poured 7 - 8 kg of an acid mixture of ordinary composition, see above), and 0.8 kg of glycerin is added to this mixture using siphons from bottles placed on the shelf; in this case, a stream of compressed air is passed into each pot through a special tube. Since 1880, they began to move to methods in which large quantities of glycerin were processed at a time, as is done at Nobel’s factories, which we will describe in more detail.
The starting materials, i.e. nitric, sulfuric and glycerin acids, must be as clean and anhydrous as possible, namely: sulfuric acid. V. 1.84 with a content of at least 95 - 96% H 2SO4, nitrogen - sp. weight 1.50 with a content of not less than 93% HNO 3 and glycerol. V. 1.26 with a content of no more than 3% water. Impurities such as significant amounts of nitrous oxide in nitric acid or the presence of fatty acids in glycerin are especially harmful, since they contribute to the initiation of dangerous oxidation reactions and interfere with the purity of washing the prepared N. The preparation of the acid mixture is carried out in large cylindrical cast iron vessels with or without stirrers inside stirrers In the latter case, the entire weighed amount of nitric acid is poured in first, and sulfuric acid in an appropriate amount is added afterwards, due to the difference in beats. weights of both acids by local heating, homogeneity of mixing is achieved by itself. The mixing proportion currently used is approx. per 1 part of nitric acid. 1.666 parts (mostly) or 2 parts (less often) sulfuric acid. From the mixing vessels, the mixture is transferred to nitration apparatus either by gravity, or using thick-walled cast iron pneumatic lifts (montejus). Relative amounts for nitration - for 1 part of glycerin from 8 to 8.5 parts of the acid mixture. This amount of the mixture is significantly greater than that required by theory, since 8 - 8.5 parts of it contain about 3 parts of HNO 3, while according to theory, only 2.05 parts of this acid are required to convert 1 part of glycerol into N. In large-scale production, nitric acid is usually prepared at the same plant from Chilean saltpeter using a spent acid mixture. The nitration of glycerol itself is carried out in a nitration apparatus (Fig. 1).
Fig. 1.
It consists of a lead vessel A
, placed in a wooden vat
B
and closed with a removable lead lid
I
, which is covered with cement during operation.
Passing through the lid are: the ends of two lead coils D
located inside the apparatus and intended to cool the mixture by means of cold water flowing through them;
tube C
, which brings compressed cold air into the apparatus for stirring during operation;
pipe F
, which removes nitric acid vapor from the apparatus;
thermometers E
, of which one reaches almost to the bottom, and the other is immersed only in the upper layer of liquid;
tube G
for pouring a measured amount of acid mixture;
tube H
for infusing glycerin, bent at the bottom of the apparatus into a ring, with small holes.
In addition, the lid has several glass windows L
for observing the phenomena occurring in the apparatus.
A similar window J
is also installed in the exhaust pipe for observing brown vapors of nitrogen oxides formed in cases of dangerous oxidation reactions or so-called development in the apparatus.
decomposition of N. Vessel M
is used to measure the amount of glycerin, determined by the indicator tube
N
, as well as to inject it into the acid mixture by means of compressed (up to 2 atmospheres) air admitted through tube
O.
Through tap
K
, the liquid is discharged from the apparatus. During operation, cold water is carried not only through the internal coil, but also through the annular space between the lead and wooden external machines. 150 kg of glycerin are processed at a time. Having let in the required amount of the acid mixture and cooled it (by passing cold compressed air and a stream of water) to 15 - 20 °, they begin spraying glycerin (at a temperature not lower than 20 °), regulating its influx so that the heating in the apparatus does not rise above 25 - 30°. If the temperature continues to rise, approaching the indicated limit even after the influx of glycerin has stopped, then the passage of cold air is increased, and if even after that the rise does not stop, then the contents of the apparatus are quickly released into a large vat of water; otherwise, decomposition of H. may easily begin, which may end in an explosion. The entire operation of processing glycerin with acids, including filling with the mixture and emptying, requires no more than 1 - 1 1/2 hours of time. Separation of N. from acids is carried out by settling their mixture in the so-called. separator (Fig. 2).
Fig. 2.
This is a lead quadrangular box with a conical bottom, inserted into the same wooden box A.
The cover contains: exhaust pipe
D
with window
E
;
tube K
for introducing the mixture from the nitration apparatus;
a hole for inserting a thermometer and several windows. The same window J
with tightly sealed glass is also located on the side wall to observe the levels of acid and floating H. From the conical bottom of the vessel there is a tube
G
with a window
F
and taps
H.
Floating of N to the surface of acids occurs more easily if it is lowered as it is released through tap
J
into the adjacent rinsing tank
L.
Presence of foreign matter, e.g.
fats in glycerin, lead sulphate, etc., makes isolation difficult. Under normal conditions, with clean materials, the operation lasts about 30 minutes. After draining almost the entire mass of N., the acidic mixture is released through one of the lower taps until
a layer of cloudy mixture consisting of impurities and various lower nitroproducts appears
F. At this moment, close the tap and release the remainder of the mixture into a suitable vessel for transfer to vat L.
Lower nitro products, formed from glycerol itself or foreign impurities, have a lower specification.
weighing compared to N., float on it in the form of foam and are especially prone to decomposition with significant heat release when exposed to air. This must be kept in mind when producing N. separation, since the presence of the said foam can cause decomposition inside the separator; then red-brown vapors appear in window E
and the temperature begins to rise by itself. In such cases, the contents are released through the third lower tap into a large vat of water, as in decompositions in a nitration apparatus.
Washing N., separated from excess acids, is done in two steps. First, it is subjected to pre-washing in the above-mentioned vat L.
This is a cylindrical lead vessel with an inclined bottom and two taps
M
, of which the lower one is intended for releasing N., and the upper one for pouring out water.
Chatting is carried out using compressed (up to 2 atmospheres) air admitted through pipe N
, which is bent at the bottom and equipped with a number of small holes. Settling, due to the large difference in beats. weights of water and N., occurs quickly. The initial water temperature should be approx. 15°, and during high tide N. should not rise above 30°. After the first wash, the second and third are performed in the same way; wash for the last time in another 2.5% soda solution, pouring this solution into a layer several centimeters thick. To completely remove the acid, N. is lowered into wooden vats lined with lead inside and similar in design to the previous vat. Using triple the volume of water, 10 to 18 washes of 15 minutes are performed here. each with stirring the mixture using compressed air (or mechanical mixers); during the 3rd and 5th washings, a 1% soda solution is taken instead of water. The most favorable water temperature is 25 - 33°; in some factories the first flush is carried out at 50°. A product is considered well washed if it passes the heat resistance test described below. The last operation during fabrication consists of filtering the N. to dehydrate and remove random solid impurities. For this purpose, it is passed through special filters (Fig. 3).
Fig. 3.
Into the lid of a wooden cylindrical vessel lined with lead A
a lead filtration cylinder
G
;
on the lower edge of its K
lies a bronze ring
L
with a mesh
M
and felt;
a layer of calcined table salt O
and another felt
P
with a lead ring
Q
;
R
is pressed onto the upper felt .
Using the J
, the filtration cylinder can be easily removed for cleaning or inspection of the inside of the vessel. Such a filter, moistened with anhydrous H., does not allow mechanically mixed (emulsified) water to pass through it, while the last traces of moisture (dissolved in N.) are absorbed by table salt; for the purpose of better drying, dried magnesium chloride is sometimes added to the latter. In the acidic liquid separated from the product, a small amount of N may form over time; therefore, this liquid, before it is put into processing to extract HNO 3 and H 2 SO 4, is subjected to preliminary settling (about a week) in large separators designed similar to those described above; pop-up N. is drained from time to time and undergoes regular washing. On the other hand, the discharged wash water also carries with it a certain amount of product mechanically; To separate and capture the finely crushed N. in these waters, they are passed through a long lead box with transverse partitions equipped with cutouts alternately, sometimes at the bottom, sometimes at the top (Fig. 4).
Fig. 4.
Regarding the N extracted during both of these operations, it should be noted that it requires especially thorough washing, since it is in it that the aforementioned harmful lower nitroproducts accumulate.
2) Pure N., prepared from completely colorless glycerin with the help of pure acids, is an oily, colorless liquid, like water, odorless at ordinary temperatures, with a sweetish, somewhat pungent taste; but complete colorlessness is rarely obtained, and its ordinary specimens are yellowish or brownish in color. Very poisonous; poisoning can occur not only by ingestion and inhalation of vapors, but also through the skin by simple touch, and is usually manifested by severe dizziness, headaches, vomiting, etc.; Deaths are possible when ingesting significant quantities (up to 10 g). In case of mild poisoning, suffering is alleviated by strong black coffee and acetic acid morphine (in very small doses), but workers in factories get used to such poisoning and, without any visible effect on health, soon cease to feel painful attacks. In the cold, N. freezes into a white crystalline mass. Its melting point increases with increasing purity: a product of ordinary purification melts at + 9° or + 10°, and recrystallized several times at + 13.3°. Ud. liquid weight 1.599, solid 1.735. Water dissolves N. in an extremely small amount (about 0.003%), but it is soluble in many organic solvents, for example. in methyl, ethyl and amyl alcohols, acetone, in ordinary and acetic ether, acetic acid, chloroform, benzene, etc. From very strong solutions in methyl alcohol, when cooled, it is possible to crystallize N. and thereby achieve its greatest purification; crystallization occurs especially easily if a ready-made crystal is dropped into a solution cooled to 0°. N. himself dissolves camphor, soluble pyroxylin (collodion) and other similar substances. In the form of solutions in methyl alcohol, Nobel, before the discovery of dynamites, proposed transporting and storing N., since in this state it is not sensitive to accidental impacts, but with the addition of water it immediately precipitates with all its properties. Vapor elasticity of N. in a void: at 15° - 5 mm, at 87° - 27 mm, at 100° - 30 mm. A two-hour wash in water heated to 50°, with compressed air passing through, produces a loss of 0.15% through evaporation. These losses can be significant if N. in heated preparations is in a finely crushed state, for example. dynamite heated at 40° - 50° C. over several days, sometimes loses up to 10%. Well washed and dried N. is a permanent substance, for an indefinitely long time - at temperatures up to 50°, and for shorter periods of time - at temperatures up to 100°. But if it is not pure enough (contains acids), it can slowly decompose even at ordinary temperatures: brown vapors of nitrogen oxides appear, the liquid turns greenish and receives a strongly acidic reaction, oxalic and other organic acids are formed, etc. In this state slow decomposition of N. easily explodes from the action of relatively weak impulses, and even by itself - due to local self-heating. With new washing, such a product that has begun to decompose cannot always be returned to its original normal state. Therefore, the completeness of N. purification during fabrication is of great importance and must be tested every time. For this purpose, two tests are usually performed - neutrality and resistance at 65°. The first is carried out in such a way that about 2 cubic meters. cm of product is shaken for some time with 10 cc. cm of water and, by separation, determine the reaction of the latter in the presence of phenolphthalein. The resistance test at 65° is carried out by placing about 3 g of the product in a glass tube with a strip of iodine-starch paper suspended on a stopper and half-moistened and determining the period of time from the beginning of inserting the equipped tube into a bath at a temperature of 65° until a brown color appears at the border of the wetted and unwetted part of the strip; such coloring, which appears due to the release of iodine from potassium iodide under the influence of the resulting nitrogen oxides, should not occur earlier than 10 or even 30 minutes. The purer the product, the longer its resistance to heat in this test [The resistance test at 65° was originally applied by Abel to pyroxylin and has since been known as the Abel test. The readings of this test depend on the conditions, namely: a) iodine-starch paper should be prepared from cleanly washed Swedish filter paper, saturating the latter with a mixture of solutions of 3 g of starch in 250 cc. cm of water and 1 g of potassium iodide per 250 cc. cm of water; after drying at ordinary temperatures, the sheets are cut into strips of 12 × 15 mm and stored in sealed dark glass jars, b) assay tubes, 14 cm long and 16 mm wide, must be tightly closed with stoppers equipped with hooks at the bottom for hanging iodine-starch papers; c) wetting the upper half of the paper strips is done with a 10% solution of pure glycerin; d) during testing, the equipped tubes are pushed into the bath so much that the iodine-starch papers are immediately above the lid of the bath]. But to fully judge the purity of N., it is also necessary to test it in relation to its composition. The latter in practice comes down to the determination of moisture and nitrogen in it. Determination of humidity is carried out by leaving a sample of N. for a long time at ordinary temp. in a void above calcium chloride, and nitrogen is usually determined using a nitrometer (see); a completely clean and dry product should contain about 18.5% nitrogen, i.e., the amount required by the formula N itself. By heating very small quantities carefully, it is possible to distill N. without decomposition, for example. placing a drop of it on a moderately heated metal plate. But if the plate is heated so much that N. immediately comes to a boil, then the drop explodes. According to Champion's experiments, very small amounts of nitrogen at high temperatures are generally contained as follows: at 185° - boiling with the release of brown vapors; at 194° - slow volatilization; at 200° - rapid volatilization; at 218° - rapid combustion; at 241° - explosion (difficult); 257° - strong explosion; at 267° - weak explosion; at 287° - an even weaker explosion with flame. At red heat, a drop of N. takes on a spheroidal state and evaporates without an explosion. According to Kopp, a faint flash occurs on a red-hot metal plate. But other phenomena occur when significant quantities of H are exposed to high temperatures. First, a slow decomposition occurs over a more or less short period of time with the release of brown vapors and the volatilization of part of the product itself, but as soon as the temperature due to self-heating or the absorption of heat from the bath rises to 180 ° (approximately), and the decomposing liquid boils, then immediately there is a strong explosion of the entire taken mass. Burning and heated bodies ignite N. very difficult: a lit match goes out in it, a hot platinum wire stops emitting light, etc. But once lit, it burns out gradually until the temperature of the rest of the mass rises to 160°, and then general explosion. Nitroglycerin explodes much more easily on impact. When an anvil is struck with a hammer, the part itself that was directly hit explodes, while adjacent parts are scattered without an explosion; the amount of work required for this is determined to be 0.75 kilograms. Frozen N. is less sensitive to shock; in this state, for an explosion, he needs to impart an impact work almost 3 times greater. The best means for exploding N. when using it is to ignite a capsule with mercury fulminate (0.1 - 0.3 g for liquid and 1 - 2 g for frozen) in direct contact with the charge. During an explosion, decomposition occurs according to the equation:
2C 3 H5(NO3)3 = 6CO2 + 5H2O + 3N2 + 0.5O2
i.e., the following are formed: water vapor, carbon dioxide, nitrogen and oxygen. They try to utilize this oxygen in dynamites (see) with an active absorber. The absence of carbon monoxide makes explosion products completely harmless, which is especially valuable for underground blasting. On the volume of gases formed, the amount of heat released, the speed of propagation of the explosion in large charges, force, etc., see respectively. article In relation to various chemical reagents, N. is contained similarly to other nitrogen esters: it is washed with alkalis, decomposed with acids to release nitric acid, and with reducing agents it is converted back into glycerol with the release of nitric oxide or ammonia. To discover the smallest quantities of N., aniline and concentrated sulfuric acid are added to the test liquid: a purple-red color is obtained, which, when diluted with water, turns green (Werber).
I. M. Cheltsov. Δ.
N. ( med.
)
.
When inhaling vapors, as well as after lubricating the tongue, N., in small doses, causes headache, nausea, dizziness, a feeling of heat and rapid heartbeat; after internal use of larger doses, headache, trembling, muscle weakness, reaching complete paralysis, shortness of breath, and, in some cases of fatal poisoning, severe respiratory distress, cyanosis, and deep depression of the nervous system were observed. Despite these dangerous properties, N., used in homeopathy for various nervous diseases (under the name “glonoina”), was recently proposed as a remedy against neuralgia, especially against attacks of angina pectoris; Further, this drug has been tested for bronchial asthma, in some cases of migraine, for epileptic seizures, and St. John's dance. Witt, and for acute and chronic inflammation of the kidneys. N. is prescribed 0.0002 - 0.001 g several times a day or in drops, from 1 to 5 drops in a 1% oil or alcohol solution; This product is also prepared in lozenges.
D.K.
Table of contents
Industrial production
In industry, the process of producing nitroglycerin has long been automated. The system that is currently used, in its main aspects, was invented back in 1935 by Biazzi (and that’s what it’s called - the Biazzi installation). The main technical solutions in it are separators. The primary mixture of unwashed nitroglycerin is first separated into two phases in the separator under the influence of centrifugal forces - the one with nitroglycerin is taken for further washing, and the acids remain in the separator.
The remaining stages of production coincide with the standard ones. That is, mixing glycerin and a nitrating mixture in a reactor (produced using special pumps, mixed with a turbine mixer, more powerful cooling using freon), several washing stages (with water and slightly alkaline water), before each of which there is a stage with a separator.
The Biazzi installation is quite safe and has a fairly high productivity compared to other technologies (however, a large amount of product is usually lost during washing).
1.5. Brief information about the main explosives
Depending on their sensitivity to external influences and their ability to transition from combustion to detonation, explosives are divided into three main groups of explosives.
Initiating or primary explosives are used to initiate detonation or combustion of explosives of other groups. Combustion and detonation of initiating explosives occurs with an insignificant expenditure of external energy as a result of thermal or mechanical effects (heating, impact, friction).
High explosives, or secondary explosives, are used to make explosive rounds for ammunition and for blasting purposes. Their combustion turns into detonation only under certain conditions (for example, when burning a large mass of a substance with a large number of pores or when burning in a closed, durable vessel). When using high explosives, detonation is caused by the explosion of an auxiliary charge of the initiating (primary) explosive or by the explosion of a charge of another high explosive.
Gunpowder, or propellant explosives, are used as propellant charges for firearms and as fuel for jet engines. In composition they are close to high explosives, but their combustion is more stable. The combustion of gunpowder does not turn into detonation even at a pressure of several thousand atmospheres.
Under certain conditions (for example, when they are exposed to a sufficiently powerful initial impulse or if their diameter is larger than the critical one), gunpowder can detonate. Some of the gunpowders have a large critical diameter, and, in addition, the detonation of gunpowders is possible only with the explosion of a powerful detonator - for these reasons, the opinion arose that gunpowders cannot detonate.
Initiating explosives
Mercury fulminate [Hg(CNO)2] is a salt of fulminate acid HCNO, mercury fulminate is a white or gray crystalline powder with a density of 4.4 g/cm3. Flash point 175 – 1800C. Easily explodes from minor impact and friction. The decomposition of fulminate mercury occurs in accordance with the equation
[Hg(CNO)2]Hg+ 2CO+N2+ 494 kJ.
It can burn, but combustion easily and quickly turns into detonation. There are known cases of detonation as a result of a falling box of dry mercury fulminate, as a result of an object falling onto scattered mercury fulminate, etc. Sensitivity to the mechanical and thermal effects of mercury fulminate decreases in the presence of water (with a content of 30% water, it does not even ignite, but can be exploded by a detonator capsule). Mercury fulminate reacts vigorously with aluminum in the presence of moisture, so it should not be stored in aluminum containers, and mercury fulminate blasting caps are not made of aluminum. Aluminum fulminates are very sensitive compounds. A similar reaction is the formation of copper fulminate, a compound sensitive to shocks. Copper capsules are protected from moisture by varnishing inside and out.
Salts of explosive acid - fulminates - are extremely dangerous, because explode when wet and even under water (especially fulminates of mercury, gold and silver). When a splash of water containing mercury fulminate dries, the solid residue explodes under the influence of sunlight. Dust, as well as all wash water and water waste from the production of fulminates, are prone to spontaneous explosion and before removal must be neutralized by heating to 90 - 950C, which is also unsafe. Fulminates are used in pyrotechnics as fuses for other explosives, for gilding (explosive gold), for the manufacture of caps and fuses. All these drugs explode from impact, falling, friction, shock, heat, flame, acids and sunlight. Mercury fulminate is used to equip primers - igniters and primers - detonators. Due to the high sensitivity of dry mercury fulminate to mechanical stress, it can only be transported in equipped products. Long-term storage of mercury fulminate in front of equipment is permitted only under water.
Lead azide [Pb(N3)2] is a salt of hydronitric acid HN3, white powder with a density of 4.8 g/cm3 and a flash point of 330-3400C. Has high sensitivity. There are known cases where lead azide exploded as a result of pressing a fingernail on its crystals. To reduce sensitivity, it is phlegmatized with paraffin. When moistened, lead azide does not lose its sensitivity. When ignited by an external heat source, it instantly detonates. Interacts with copper, does not interact with aluminum. Lead azide is used to fill detonator caps. Hydronitric acid HN3 in anhydrous form can explode even by simply shaking the vessel. In a dilute aqueous solution during storage, it practically does not decompose. Its vapors are very poisonous, solutions cause inflammation of the skin.
The explosive decomposition of hydronitric acid follows the equation: HN3H2+ 3N2+ 590 kJ
Lead trinitroresorcinate (TNRS) [C6H(NO2)3(O2Pb)H2O] is a yellow-brown powder with a density of 3.1 g/cm3 and a flash point of 2750C. The impact sensitivity is lower than lead azide and the ignition sensitivity is higher. Used for equipping igniter primers.
Tetrazene or guanylnitrosoaminoguanyltetrazene [C2H8ON10] NH2 NH–NH-NO NH=C–NH–N=N–C=NH
Fine-crystalline yellowish powder with a density of 1.65 g/cm3 and a flash point of about 1400C. Slightly hygroscopic. It is close in sensitivity to mercury fulminate. Does not interact with metals.
Home conditions
Unfortunately, although, rather, fortunately, the synthesis of nitroglycerin at home is associated with too many difficulties, overcoming which is generally not worth the result.
The only possible method of synthesis at home is to obtain nitroglycerin from glycerin (as in the laboratory method). And here the main problem is sulfuric and nitric acids. The sale of these reagents is permitted only to certain legal entities and is strictly controlled by the state.
The obvious solution is to synthesize them yourself. Jules Verne in his novel “The Mysterious Island,” talking about the episode of the production of nitroglycerin by the main characters, omitted the final moment of the process, but described in great detail the process of obtaining sulfuric and nitric acids.
Those who are really interested can look into the book (the first part, chapter seventeen), however, there is a catch here - the uninhabited island was literally replete with the necessary reagents, so the heroes had at their disposal sulfur pyrites, algae, a lot of coal (for burning), potassium nitrate, and so on. Will the average addicted person have this? Hardly. Therefore, homemade nitroglycerin in the vast majority of cases remains just a dream.
Application
I drink it in the smallest doses, I drop the solution onto the sugar, and it is capable of throwing any of the nearby mountains into the air.
It, dissolved in gelatin and turned into dynamite, will thunder in a distant golden valley, exploding the rocks.
And the fuse cord shuddered, the primer fired, and it breaks the hard, indestructible mineral.
He is the cause of heartache, And he is the only medicine for me - This is how medicine explained In the cold mountain side.
V.T. Shalamov "Nitroglycerin"
In pharmacology
Nitroglycerin belongs to the category of substances called vasodilators - drugs that lower blood pressure, relaxes the smooth muscles of blood vessels, bronchi, biliary and urinary tracts, and the gastrointestinal tract. Its main use is for angina pectoris, mainly for relieving acute attacks of spasms of the coronary vessels. It is of little use for preventing attacks due to its short duration of action. Sometimes used for embolism of the central retinal artery, as well as functional cholecystopathy.
Used in the form of 0.5 mg tablets to be placed under the tongue; and also in a 1% alcohol solution.
Nitroglycerin is included in small doses in the Zanifil gel used in Durex CSD500 condoms to stimulate an erection during sexual intercourse.
In explosives
Nitroglycerin was widely used in explosives. In its pure form it is very unstable and dangerous. After Sobrero's discovery of nitroglycerin, in 1853 the Russian chemist Zinin proposed using it for technical purposes. 10 years later, engineer Petrushevsky was the first to begin producing it in large quantities; under his leadership, nitroglycerin was used in mining in 1867. Alfred Nobel in 1863 invented an injector-mixer for the production of nitroglycerin and a detonator capsule, and in 1867 - dynamite, obtained by mixing nitroglycerin with kieselguhr (diatomite, infusor earth).
In literature and cinema
- The hero of the adventure novel “The Mysterious Island” (1874) by Jules Verne uses nitroglycerin to undermine a granite rock. The author describes in detail the process of obtaining nitroglycerin from natural substances found on the island (although Jules Verne deliberately omitted one of the important stages of the synthesis). The writer characterizes this substance as follows:
Indeed, it was nitroglycerin - a terrible substance, ten times more explosive than gunpowder, and which had already caused so much misfortune. True, since they learned how to turn nitroglycerin into dynamite by mixing it with some porous substance - for example, clay or sugar, capable of holding a dangerous liquid, it can be used with less risk. But at the time the colonists were operating on Lincoln Island, dynamite was not yet known.
- Much of the plot of The Wages of Fear (1953) revolves around the process of transporting nitroglycerin by truck.
- In Chuck Palahniuk's novel Fight Club (1996) and the film of the same name (1999), the protagonist obtains nitroglycerin by saponifying human fat and adding the resulting glycerin to a mixture of nitric acid and sulfuric acid.
Take one part of 98% fuming nitric acid and mix with three parts of concentrated sulfuric acid. This should be done in an ice bath. Then add glycerin drop by drop from an eye dropper. You received nitroglycerin.
— Ch. Palahniuk “Fight Club”
- In the film “The Vertical Limit” (2000) there is an episode with the spontaneous detonation of liquid nitroglycerin from direct exposure to sunlight (but nitroglycerin cannot explode from thermal exposure, but burns like alcohol. Only mechanical action can make nitroglycerin explode - editor's note).
- In the TV series "Prison Break" season 2 episode 9, a box with ampoules of nitroglycerin, which Michael Scofield hid, is found in the botanical garden.
- In the series "Lost" season 1 episode 24-25, dynamite (nitroglycerine stabilized by a porous substance) is found on the ship "Black Rock"
- In the film The Legend of Zorro (2005), the main villain demonstrates nitroglycerin to customers, and the final scene of the film takes place on a train transporting nitroglycerin.
- In the serial film “Terrorist Ivanova,” Polina Ivanova wants to take revenge on the investigator for the death of her husband by blowing up the police station with nitroglycerin.
- In Sergio Leone's film A Fistful of Dynamite, one of the main characters, an Irish terrorist (James Coburn), is hung with sticks of dynamite and bottles of nitroglycerin. At the beginning of the film, he demonstrates the latter's explosive properties by dropping a drop onto a rock.