Contraindications
hypersensitivity to the components of the drug;
liver diseases;
alcoholism;
traumatic brain injury;
brain diseases;
epilepsy;
chronic heart failure (in the stage of decompensation);
glucose-galactose malabsorption;
sucrase/isomaltase deficiency;
fructose intolerance;
pregnancy;
breastfeeding period;
children's age (up to 3 years).
With caution: diabetes mellitus; children over 3 years of age (due to the presence of ethanol in the drug).
Pertussin-K 100 ml syrup
APPROVED by the order of the Chairman of the Committee for Control of Medical and Pharmaceutical Activities of the Ministry of Health and Social Development of the Republic of Kazakhstan dated “___”_______________201_ No._____ Instructions for the medical use of the drug Pertussin-K Trade name Pertussin-K International nonproprietary name No Dosage form Syrup Composition 100 ml of syrup contain active substances: thyme liquid extract 12.0 g excipients: potassium bromide, ethanol 80%, sugar syrup. Description Dark brown liquid with an aromatic odor and sweet taste. Pharmacotherapeutic group Expectorants. ATC code R05CA Pharmacological properties A combined preparation of plant origin, has an expectorant effect, determined by the content of essential oils, which is enhanced in combination with potassium bromide. Pertussin-K has pronounced expectorant and anti-inflammatory properties, stimulates the motor activity of the ciliated epithelium of the upper respiratory tract, increases the amount of secretory secretion from the mucous membranes and causes liquefaction of sputum and acceleration of its excretion. The antispasmodic effect of the drug is due to the presence of thyme and flavonoids in the herb. Potassium bromide reduces the excitability of the central nervous system. Indications for use - diseases of the upper respiratory tract accompanied by cough - tracheitis - bronchitis (as an expectorant and cough softener) - whooping cough Method of administration and dosage For oral administration. Shake before use. The syrup is used orally 3 times a day. Adults: 1 tablespoon 3 times a day. The course of treatment is 5-7 days. After opening, store the drug for no more than 14 days. Increasing the duration and conducting repeated courses of treatment is possible on the recommendation of a doctor. Side effects - allergic reactions (skin rash) - heartburn, nausea, vomiting Contraindications - hypersensitivity - decompensated chronic heart failure - pregnancy, lactation - children under 18 years of age - liver, kidney diseases - alcoholism - brain diseases - epilepsy, cranial brain injury - peptic ulcer of the stomach and duodenum Drug interactions If necessary, Pertussin-K is prescribed in combination with antitussives. Special instructions With caution in case of diabetes mellitus Features of the drug's influence on the ability to drive vehicles and potentially dangerous mechanisms Care should be taken when driving vehicles or moving mechanisms, because the drug contains ethyl alcohol. Overdose Not identified Release form and packaging 100 ml of the drug in bottles made of glass with a screw neck, sealed with polyethylene or universal (aluminum) caps or plastic or in bottles made of dark brown opaque polyvinyl chloride, polyethylene terephthalate with a screw neck with a capacity of 100 ml, sealed with screw caps from high-density polyethylene or in dark pink opaque polyvinyl chloride, polyethylene terephthalate screw-neck bottles with a capacity of 125 ml, sealed with screw caps. The bottles, along with instructions for medical use in the state and Russian languages, are placed in cardboard boxes. Storage conditions Store in a place protected from light, at a temperature not exceeding 25 ° C. Keep out of the reach of children ! Shelf life 3 years Do not use after the expiration date Dispensing conditions Without a prescription Name and address of the manufacturer Pharmaceutical, Republic of Kazakhstan Aktobe, s/o Novy, uch. No. 467, telephone/fax Owner of the registration certificate Pharmaceutical, Republic of Kazakhstan Address of the organization that accepts claims from consumers on the quality of products (goods) on the territory of the Republic of Kazakhstan Pharmaceutical, Republic of Kazakhstan, Aktobe, s/o Novy, uch. No. 467, telephone/fax Email address
special instructions
The drug contains 8–11% ethanol. The absolute alcohol content is: in 1 teaspoon (5 ml) - up to 0.43 g, 1 teaspoon (10 ml) - up to 0.87 g, 1 tablespoon (15 ml) - up to 1.3 g The maximum daily dose of the drug for adults - 3 tablespoons (45 ml) - contains up to 3.9 g of absolute alcohol.
During the period of use of the drug, care must be taken when performing potentially hazardous activities that require increased concentration and speed of psychomotor reactions (driving vehicles, working with moving mechanisms, working as a dispatcher and operator, etc.).
Instructions for patients with diabetes: the sucrose content in 1 teaspoon (5 ml) of the drug corresponds to approximately 0.32 XE, 1 teaspoon (10 ml) - approximately 0.64 XE; 1 tablespoon - approximately 0.96 XE.
Treatment regimens and doses: instructions
The syrup must be taken several times a day at intervals of 4–6 hours, measuring the required volume using a spoon:
- children 3–6 years old: 1 tsp. (2.5 ml) three times a day;
- from 6 to 12 years: 2 tsp. or 1 tbsp. l. 2–4 times a day, depending on the severity of the symptoms of the disease, the daily dose of the medicine is about 10 ml;
- teenagers and adults are allowed to drink 1.5 tbsp. l. syrup three times a day or 1 tbsp. l. 4–6 times, while the daily volume of syrup should not exceed 45 ml.
Pertussin does not require additional dilution; its sweet taste allows very young patients to drink it without disgust. Recommended time of use: 30–60 minutes after meals, do not take the syrup on an empty stomach.
The duration of treatment depends on the severity of inflammation of the respiratory tract and is 7–10 days. The maximum course of taking Pertussin without a break is 2 weeks.
The medicine requires careful use in cases of carbohydrate metabolism disorders or diabetes, as it contains sugar. Adults should stop driving immediately after consuming the product due to the presence of ethyl alcohol in the syrup.
Note!
Description of the drug Pertussin syrup fl. 100g on this page is a simplified author’s version of the apteka911 website, created on the basis of the instructions for use.
Before purchasing or using the drug, you should consult your doctor and read the manufacturer's original instructions (attached to each package of the drug). Information about the drug is provided for informational purposes only and should not be used as a guide to self-medication. Only a doctor can decide to prescribe the drug, as well as determine the dose and methods of its use.
Pertussin-Ch
Registration number: Р№ LSR-008022/09
Trade name of the drug: Pertussin-Ch.
Dosage form: Syrup.
Composition per 100 g: Active substances:
- Thyme liquid extract – 12.0 g
- Potassium bromide - 1.0 g
Excipients:
- Sucrose solution 64% (sugar syrup) - 82.0 g
- Ethanol (ethyl alcohol) 95% - 4.06 g
- Purified water - 0.94 g
Description: Thick brown liquid with an aromatic odor.
Pharmacotherapeutic group: Expectorant.
ATX code: R05CA
Pharmacological properties: Combined drug. Thyme herb extract has an expectorant effect, increases the amount of secretory secretion from the mucous membranes of the upper respiratory tract, helps to liquefy mucus and accelerate its evacuation. Potassium bromide reduces the excitability of the central nervous system.
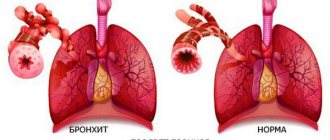
Indications for use: As an expectorant in the complex therapy of acute respiratory diseases. Tracheitis, bronchitis, and also with whooping cough.
Contraindications: Hypersensitivity to the components of the drug, liver disease, alcoholism, traumatic brain injury, brain diseases, epilepsy, chronic heart failure (in the stage of decompensation), pregnancy, lactation, children (up to 3 years).
With caution: Diabetes mellitus. Children over 3 years of age (due to the presence of ethanol in the drug).
Directions for use and dosage: The drug is taken orally, after meals (due to the possibility of decreased appetite). Adults: 1 tablespoon 3 times a day; children from 3 to 6 years old - 1/2 - 1 teaspoon 3 times a day; children from 6 to 12 years old - 1 - 2 teaspoons 3 times a day; children over 12 years old - 1 dessert spoon 3 times a day. The course of treatment is 10 - 14 days. Increasing the duration and conducting repeated courses of treatment is possible on the recommendation of a doctor.
Side effects: Possible allergic reactions, heartburn.
Overdose: Symptoms: In case of overdose of the drug, nausea is possible. Treatment: Symptomatic.
Interaction with other drugs: The drug should not be used simultaneously with antitussive drugs, as this makes it difficult to cough up liquefied sputum.
Special instructions: The drug contains 8 - 11% ethanol. The absolute alcohol content is: in 1 teaspoon (5 ml) - up to 0.43 g, in 1 dessert spoon (10 ml) - up to 0.87 g, in 1 tablespoon (15 ml) - up to 1.3 g. The maximum daily dose of the drug for adults—3 tablespoons (45 ml)—contains up to 3.9 g of absolute alcohol. During the treatment period, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Instructions for patients with diabetes mellitus: The sucrose content in 1 teaspoon (5 ml) of the drug corresponds to approximately 0.22 XE, in 1 dessert spoon (10 ml) - approximately 0.44 XE; in 1 tablespoon - approximately 0.66 XE.
Release form: Syrup. 100 g, 125 g of the drug in orange or brown glass bottles, sealed with aluminum caps with perforations or screw caps. Each bottle, along with instructions for use, is placed in a cardboard pack. It is allowed to pack bottles without a pack together with an equal number of instructions for use in a group package.
Storage conditions: In a dry place at a temperature of 12 to 15 °C. Keep out of the reach of children.
Shelf life: 4 years. Do not use after expiration date.
Conditions for dispensing from pharmacies: Without a prescription.
Side effects
With prolonged use, bromism phenomena are possible: skin rash, rhinitis, conjunctivitis, gastroenterocolitis, general weakness, ataxia, bradycardia. Treatment of bromism consists of stopping the drug and increasing the elimination of bromine salts (prescribing large amounts of sodium chloride, fluids, diuretics).
Gastrointestinal disturbances (including nausea, vomiting, diarrhea) are possible.
Allergic reactions to the components of the drug are possible.
If any adverse events occur, you should consult a doctor.
Pertussin-ECO syrup (flask 100g (individual pack with measuring spoon))
A country
Russia
The country of production may vary depending on the batch of goods. Please check with the operator for detailed information when confirming your order.
Compound
Per 100 g: Active ingredients: Liquid thyme extract - 12g Potassium bromide - 1g Excipients: Sucrose solution 64% - 82g Ethanol (ethyl alcohol 95%) - 3.975g Purified water - 1.025g
pharmachologic effect
Combined drug. Thyme herb extract has an expectorant effect, increases the amount of secretory secretion from the mucous membranes of the upper respiratory tract, helps to liquefy mucus and accelerate its evacuation. Potassium bromide reduces the excitability of the central nervous system.
Indications for use
As an expectorant in the complex treatment of acute respiratory diseases, tracheitis, bronchitis, as well as in children with whooping cough.
Interaction
The drug should not be used simultaneously with antitussive drugs, as this makes it difficult to cough up liquefied sputum.
Side effect
Allergic reactions and heartburn are possible.
Contraindications
Hypersensitivity to the components of the drug, liver disease, alcoholism, traumatic brain injury, brain diseases, epilepsy, chronic heart failure (in the stage of decompensation), pregnancy, breastfeeding, fructose intolerance, sucrose/isomaltose deficiency, glucose-galactose malabsorption, children's age (up to 3 years). With caution: diabetes mellitus; children over 3 years of age (due to the presence of ethanol in the drug).
special instructions
The drug contains 8-11% ethanol. The absolute alcohol content is: in 1 teaspoon (5 ml) up to 0.43 g, in 1 dessert spoon (10 ml) - up to 0.87 g, in 1 tablespoon (15 ml) - up to 1.3 g. The maximum daily dose of the drug for adults is 3 tablespoons (45 ml) - it contains up to 3.9 g of absolute ethyl alcohol. During the treatment period, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions. Instructions for patients with diabetes: the sucrose content in 1 teaspoon (5 ml) of the drug corresponds to approximately 0.32 XE, in 1 dessert spoon (10 ml) - approximately 0.64 XE; in 1 tablespoon - approximately 0.96 XE.