Pharmacological properties of the drug Lazolvan
Ambroxol, the active ingredient in Lazolvan, increases the secretion of the glands of the respiratory tract. Ambroxol enhances the secretion of pulmonary surfactant and stimulates ciliary activity. This leads to improved mucus separation and elimination (mucociliary clearance). Activation of fluid secretion and increased mucociliary clearance facilitate the removal of bronchial secretions and reduce cough. The local anesthetic effect of ambroxol can be explained by its sodium channel blocking properties. In vitro studies have demonstrated that ambroxol reversibly and dose-dependently blocks neuronal sodium channels. These pharmacological properties, leading to a rapid reduction in the severity of pain and pain-related discomfort in the nasal cavity and trachea, are consistent with data from additional observation of symptoms in clinical studies of the effectiveness of ambroxol in the treatment of the upper respiratory tract. In vitro studies have demonstrated that the drug reduces the number of cytokines, as well as the number of tissue-associated mononuclear cells and polymorphonuclear cells. Pharmacokinetics. Absorption of all oral forms of ambroxol is rapid and fairly complete, with a linear dependence in the therapeutic range. The maximum level in blood plasma is reached after 0.5–3 hours. In the therapeutic range, about 90% of the drug binds to blood plasma proteins. When administered orally, the distribution of ambroxol from the blood to the tissues is rapid, with a high concentration of the active substance in the lung tissue. Clinical studies have shown that CYP 3A4 is the dominant isoenzyme that metabolizes ambroxol. Ambroxol is metabolized mainly in the liver by conjugation. The half-life from blood plasma is 10 hours. The total clearance is within 660 ml/min, renal clearance is about 8% of the total clearance. No evidence was found that age and gender affect the pharmacokinetics of ambroxol within clinically significant limits, so there is no need for any dose adjustment.
Lazolvan Junior 15 mg/5 ml 100 ml syrup
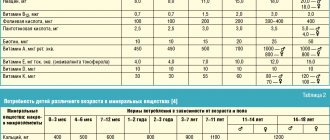
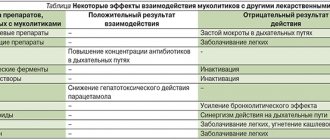
Instructions for medical use of the drug LAZOLVAN® Junior LAZOLVAN® Trade name LAZOLVAN® Junior LAZOLVAN® International nonproprietary name Ambroxol Dosage form Syrup 15 mg/5 ml, 100 ml Syrup 30 mg/5 ml, 100 ml, 200 ml Composition 5 ml syrup ( 15 mg/5 ml) contain the active substance - ambroxol hydrochloride 15 mg, excipients: benzoic acid, hydroxyethylcellulose, acesulfame potassium, liquid sorbitol (non-crystallizing), glycerin 85%, flavoring "Woodberry PHL-132195", flavoring "Vanilla 201629", purified water. 5 ml of syrup (30 mg/5 ml) contain the active substance - ambroxol hydrochloride 30 mg, excipients: benzoic acid, hydroxyethylcellulose, acesulfame potassium, liquid sorbitol (non-crystallizing), glycerin 85%, flavor "Strawberry PHL-132200", flavor " Vanilla 201629", purified water. Description Transparent or almost transparent, colorless or almost colorless, slightly viscous liquid, with a berry aroma (for a dosage of 15 mg/5 ml). Transparent or almost transparent, colorless or almost colorless, slightly viscous liquid, with a berry aroma (for a dosage of 30 mg/5 ml). Pharmacotherapeutic group Drugs to eliminate the symptoms of colds and coughs. Expectorants. Mucolytics. Ambroxol. ATX code R05CB06 Pharmacological action Pharmacokinetics Absorption. Absorption is high and almost complete, linearly dependent on the therapeutic dose. The maximum plasma concentration is achieved within 1 - 2.5 hours. Distribution. Distribution is rapid and widespread, with highest concentrations in lung tissue. Distribution volume approximately 552 l. The binding to plasma proteins is approximately 90%. Metabolism and excretion. Approximately 30% of an ingested dose undergoes a first-pass effect through the liver. CYP3A4 is the main enzyme responsible for the metabolism of ambroxol, under the influence of which conjugates are formed, mainly in the liver. The half-life is 10 hours. Total clearance: within 660 ml/min, renal clearance accounts for 83% of the total clearance. Excreted by the kidneys: 26% in the form of conjugates, 6% in free form. Excretion is reduced in case of liver dysfunction, which leads to an increase in plasma levels by 1.3-2 times, but does not require dose adjustment. Gender and age do not have a clinically significant effect on the pharmacokinetics of ambroxol and do not require dose adjustment. Food intake does not affect the bioavailability of ambroxol hydrochloride. Pharmacodynamics LAZOLVAN has secretolytic and expectorant effects; stimulates the serous cells of the glands of the bronchial mucosa, increases the content of mucous secretion and the release of surfactant in the alveoli and bronchi; normalizes the disturbed ratio of serous and mucous components of sputum. By activating hydrolyzing enzymes and enhancing the release of lysosomes from Clara cells, it reduces the viscosity of sputum. Increases the motor activity of the cilia of the ciliated epithelium, increases the mucociliary transport of sputum. Increasing secretion and mucociliary clearance improves sputum production and relieves cough. It has been proven that the local anesthetic effect of ambroxol is due to dose-dependent blockade of sodium channels of neurons. Under the influence of ambroxol, the release of cytokines from the blood, as well as from tissue mononuclear cells and polymorphonuclear cells, is significantly reduced. Clinical studies on patients with sore throat have shown a significant reduction in sore throat and redness. Indications for use - secretolytic therapy of acute and chronic bronchopulmonary diseases, characterized by impaired secretion and difficult sputum discharge. Method of administration and dosage Syrup 30 mg/5 ml: This regimen is suitable for the treatment of acute diseases of the respiratory tract and for the initial treatment of chronic conditions. The course is 14 days. Adults and children over 12 years of age: 10 ml (2 measuring cups) 2 times a day; Children from 6 to 12 years: 2.5 ml (½ measuring cup) 2-3 times a day; Children from 2 to 5 years: 1.25 ml (¼ measuring cup) 3 times a day; Children from 1 to 2 years: 1.25 ml (¼ measuring cup) 2 times a day. After 14 days, the dose can be halved. Syrup 15 mg/5 ml: Adults and children over 12 years of age: 10 ml (2 measuring cups) 3 times a day; Children from 6 to 12 years old: 5 ml (1 measuring cup) 2-3 times a day; Children from 2 to 5 years: 2.5 ml (½ measuring cup) 3 times a day; Children under 2 years: 2.5 ml (½ measuring cup) 2 times a day. General information. The drug can be taken regardless of meals. If the condition does not improve with the treatment of acute respiratory diseases, you should seek medical help. Side effects Gastrointestinal disorders Common (≥ 1/100 - < 1/10): - nausea, change in taste, decreased sensitivity in the mouth and pharynx (oral and pharyngeal hyposthesia) Uncommon (≥ 1/1,000 - < 1/100) : - vomiting, diarrhea, dyspepsia, abdominal pain, dry mouth Rare (≥ 1/10,000 - < 1/1,000): - dry throat Immune system disorders Not known: - anaphylactic reactions, including anaphylactic shock Skin and subcutaneous tissue disorders Rarely (≥ 1/10,000 - < 1/1,000): - rash, urticaria, Not known: - itching and other hypersensitivity reactions, angioedema. Contraindications - hypersensitivity to ambroxol hydrochloride or other components of the drug - rare hereditary fructose intolerance Drug interactions No clinically significant adverse interactions with other drugs have been reported. Combined use with antitussive drugs leads to difficulty in sputum discharge due to cough suppression. Increases the penetration and concentration in bronchial secretions of amoxicillin, cefuroxime and erythromycin. Special instructions Very rare cases of severe skin lesions, such as Stevens-Johnson syndrome and toxic epidermal necrolysis, have been reported with the use of ambroxol hydrochloride. They are mainly due to the severity of the underlying disease and concomitant treatment. In addition, in the early stages of Stevens-Johnson syndrome and toxic epidermal necrolysis, patients may show signs of the onset of a nonspecific disease, with the following symptoms: fever, pain throughout the body, rhinitis, cough and sore throat. The appearance of these signs may lead to unnecessary symptomatic treatment with cold medications. If skin lesions appear, the patient is immediately examined by a doctor, and ambroxol hydrochloride is stopped. For patients with decompensated renal failure, the use of LAZOLVAN is indicated only after consultation with a doctor. 5 ml of syrup contains 1.2 sorbitol, which is 4.9 g of sorbitol in the maximum recommended daily dose of 20 ml (for a dosage of 30 mg/5 ml) or 7.4 g of sorbitol in the maximum recommended daily dose of 30 ml (for a dosage of 15 mg/5 ml). This drug should not be taken by patients who have a rare congenital fructose intolerance. The drug may also have a mild laxative effect. Fertility, pregnancy and lactation Ambroxol hydrochloride penetrates the placental barrier. Preclinical studies have not shown direct or indirect negative effects on pregnancy, fetal development, childbirth or postnatal development. It is not recommended to use LAZOLVAN during the first trimester of pregnancy. Use with caution in the II–III trimesters of pregnancy and during lactation. LAZOLVAN passes into breast milk; however, when prescribed in therapeutic doses, it does not have a negative effect on the child. Preclinical studies have not shown direct or indirect negative effects on fertility. Features of the drug's effect on the ability to drive a vehicle or potentially dangerous machinery There are no reports of cases of the drug influencing the ability to drive a car or operate machinery in the post-marketing period. No studies have been conducted. Overdose Symptoms: nausea, vomiting, diarrhea, dyspepsia. Treatment: symptomatic therapy. Release form and packaging 100 ml in brown glass bottles sealed with a screw-on white plastic cap with tamper evident. The bottle comes with a plastic measuring cup with volume markings and the company logo. 1 bottle with instructions for medical use in the state and Russian languages is placed in a cardboard box (for a dosage of 15 mg/5 ml). 100 ml and 200 ml in brown glass bottles sealed with a screw-on white plastic cap with tamper evident. The bottle comes with a plastic measuring cup with volume markings and the company logo. 1 bottle with instructions for medical use in the state and Russian languages is placed in a cardboard box (for a dosage of 30 mg/5 ml). Storage conditions Store at temperatures up to 30°C. Do not freeze! (for dosage 15 mg/5 ml). Store at temperatures from 15°C to 30°C (for a dosage of 30 mg/5 ml). Keep out of the reach of children! Shelf life 3 years. Use within 1 year after opening. Do not use after the expiration date stated on the package. Conditions for dispensing from pharmacies Without a prescription Manufacturer Boehringer Ingelheim Espana S.A., Spain Name and country of the owner of the registration certificate Boehringer Ingelheim International GmbH Binger Strasse 173, 55216 Ingelheim, Germany Address of the organization receiving claims from consumers regarding product quality in the territory of the Republic of Kazakhstan Representative office "Boehringer Ingelheim Pharma Ges mbH" in the Republic of Kazakhstan Legal address: Almaty, 050010, st. Kairbekova 38 Actual address: Almaty, 050008, Abay Ave. 52 Business, 7th floor tel: +7 (727) 250 00 77 fax e-mail
Use of the drug Lazolvan
The following dosage regimen is recommended: tablets. Adults and children over 12 years of age: 1 tablet 3 times a day. The therapeutic effect can be increased by using 2 tablets 2 times a day. The tablets should be taken after meals and washed down with water. The duration of treatment depends on the nature of the disease and the effectiveness of the therapy. Syrup. Adults and children over 12 years of age: 10 ml (2 teaspoons) 2 times a day. The syrup is recommended to be consumed with meals. Treatment period is up to 14 days. You should consult your doctor if symptoms of the disease last more than 14 days and/or worsen despite taking Lazolvan syrup. Capsules. Adults: 1 capsule is the daily dose; it should be taken in the morning or evening after meals. Capsules must be swallowed whole, without chewing, with plenty of liquid. Treatment period is up to 14 days. You should consult your doctor if symptoms of the disease last more than 14 days and/or worsen despite taking Lazolvan Retard capsules.
Side effects of the drug Lazolvan
As a rule, taking Lazolvan is well tolerated. Gastrointestinal disorders: mild manifestations of heartburn, dyspepsia, nausea, vomiting, diarrhea; disorders of the immune system, skin and subcutaneous tissues: skin rash, urticaria, angioedema, anaphylactic reactions (including anaphylactic shock) and allergic reactions. If lesions of the skin or mucous membranes progress, you should immediately consult a doctor and stop treatment with ambroxol. Severe skin lesions, such as Stevens-Johnson and Lyell syndromes, have been reported extremely rarely in association with the use of mucolytic agents such as ambroxol. As a rule, they could be explained by the severity of the underlying disease or the use of concomitant medications.
Special instructions for the use of the drug Lazolvan
Use during pregnancy and lactation. Ambroxol penetrates the placental barrier. Animal studies have not revealed direct or indirect negative effects on pregnancy, embryo/fetal development, childbirth or postnatal development. Extensive clinical experience with use after the 28th week of pregnancy has found no evidence of negative effects on the fetus. Despite this, the usual precautions should be followed when using medications during pregnancy. The use of Lazolvan is not recommended, especially in the first trimester of pregnancy. Ambroxol passes into breast milk. Therefore, Lazolvan is not recommended to be taken during breastfeeding. However, one should not expect a negative effect on an infant if the mother takes Lazolvan during breastfeeding. Lazolvan tablets contain 684 mg of lactose in the maximum recommended daily dose (120 mg). Patients with a rare form of hereditary galactose intolerance, Lapp lactose intolerance or glucose-galactose malabsorption should not take this drug. Lazolvan syrup does not contain sugar, so it can be prescribed to patients with diabetes; the syrup does not contain alcohol. Children under 12 years of age are recommended to use Lazolvan in the form of syrup. Residues of gelatin capsules, sometimes detected in feces, do not indicate the ineffectiveness of the drug (the active substance is released from the capsules during passage through the digestive tract). This dosage form, namely Lazolvan Retard extended-release capsules, should not be prescribed to children.
Tablets 30 mg
Use in children
According to the instructions, Lazolvan® cough tablets are intended for adults and children over 6 years of age. Such age restrictions are associated with the dosage form and dosage of the active substance, which contains 30 mg in one tablet. Children under 6 years of age need less than 30 mg of the drug at a time, and it is easier for them to drink a solution or syrup than to swallow a tablet. 1 ml of solution contains 7.5 mg of active substance, which is 4 times less than in a tablet. The active ingredient in syrup is 15 mg/5 ml.1
During lactation
Taking Lazolvan® tablets during lactation is contraindicated. Its active ingredient ambroxol hydrochloride can pass into breast milk, the baby will receive a dose of the drug that he does not currently need.1
Use during pregnancy
Lazolvan® tablets are not recommended for use in the first trimester of pregnancy. Ambroxol penetrates the placental barrier, although preclinical studies have not revealed direct or indirect adverse effects on pregnancy; precautions must be taken during this period.1 This is the time when the body of the unborn child is forming and most medications are not recommended for use during this period.3
In the second and third trimester of pregnancy, it is recommended to prescribe Lazolvan® tablets only if treatment of the mother is more important than the potential risks to the fetus.1
For impaired renal function
For kidney diseases and disorders of their function, including failure, Lazolvan® tablets are used only as prescribed by a healthcare professional.
The fact is that any kidney disease, and especially renal failure, significantly changes the processes of renal filtration and distribution of drugs, purification of blood, lymph, and intercellular fluid from them. Kidney disease also affects the binding of drugs to plasma proteins, from where they are distributed to organs. Such changes may require monitoring by a healthcare professional to monitor the body's response, adjusting dosages and/or frequency of dosing.2, 3
For liver dysfunction
Liver diseases and liver failure can affect its important functions: detoxification, cleansing, metabolic.
In case of liver failure, Lazolvan® tablets should be used with caution.1, 3
Active substance
Ambroxol hydrochloride 30 mg is the active ingredient in Lazolvan® tablets. One tablet contains 30 mg of ambroxol.
It is a mucolytic, expectorant, and thins mucus. Increases secretion in the respiratory tract. Increasing mucociliary clearance improves sputum discharge and relieves cough. In patients with COPD, there was a significant reduction in the duration of exacerbations and the number of days of antibiotic therapy.1