| Composition and release form How the drug works When prescribed (indications) How to use (dosage) Contraindications for use Side effects of use | Overdose symptoms Drug interactions What to pay attention to Analogues of Xalatamax Prices in pharmacies Reviews from people who have used it |
Xalatamax is a solution of eye drops to reduce intraocular pressure. It is used in ophthalmology in the treatment of open-angle glaucoma and intraocular hypertension.
pharmachologic effect
Latanoprost in Xalatamax solution is a synthetic analogue of prostaglandin F2, with the ability to reduce intraocular pressure by increasing the outflow of aqueous humor and have a general antiglaucoma effect. The action of latanoprost is aimed at increasing uveoscleral outflow without affecting the production of moisture and the blood-ophthalmic barrier. Intraocular pressure begins to decrease 3-4 hours after administration of the drug, the maximum effect occurs after 8-12 hours, the effect lasts up to 24 hours.
Xalatamax 0.005% eye drops bottle 2.5 ml No. 3
A country
Croatia
The country of production may vary depending on the batch of goods. Please check with the operator for detailed information when confirming your order.
Compound
Bottle 2.5 ml Latanoprost 0.05 mg per 1 ml. Excipients: benzalkonium chloride - 0.2 mg, sodium dihydrogen phosphate monohydrate - 4.6 mg, sodium hydrogen phosphate - 4.74 mg, sodium chloride - 4.1 mg, purified water - 996.31 mg. Eye drops 0.005% transparent , colorless.
pharmachologic effect
Antiglaucoma drug for local use. Is Latanoprost a prostaglandin F2 analogue? and a selective FP receptor agonist. Reduces intraocular pressure by increasing the outflow of aqueous humor and has an antiglaucoma effect. The main mechanism of action of latanoprost is associated with an increase in uveoscleral outflow. It does not have a significant effect on the production of aqueous humor and does not affect the blood-ophthalmic barrier. The decrease in intraocular pressure begins 3-4 hours after administration of the drug, the maximum effect is observed after 8-12 hours, the duration of action is at least 24 hours.
Indications for use
- open-angle glaucoma; - increased intraocular pressure.
Mode of application
The drug is instilled into the conjunctival sac of the eyes, 1 drop 1 time/day, in the evening. If a dose is missed, the next dose is administered as usual (i.e., the dose is not doubled). With more frequent administration of the drug, its effectiveness decreases. The duration of the course of treatment and the possibility of its repetition are determined by the doctor.
Interaction
Latanoprost has an additive effect in reducing intraocular pressure when used in combination with beta-blockers, adrenergic agonists, carbonic anhydrase inhibitors and a partial additive effect when used in combination with m-cholinomimetics. In vitro studies have revealed that when mixed with latanoprost, eye drops containing thiomersal, precipitation occurs. Therefore, eye drops containing these substances should be used at intervals of at least 5 minutes. Simultaneous use of 2 prostaglandin analogues can cause a paradoxical increase in intraocular pressure.
Side effect
From the organ of vision: eye irritation (burning sensation, feeling of sand in the eyes, itching, tingling and foreign body sensation), blepharitis, conjunctival hyperemia, eye pain, increased pigmentation of the iris, transient pinpoint erosion of the epithelium, swelling of the eyelids, swelling and erosion of the cornea , conjunctivitis, elongation, thickening, increase in the number and intensification of pigmentation of eyelashes and vellus hair, iritis/uveitis, keratitis, macular edema (including cystoid), change in the direction of eyelash growth, sometimes causing eye irritation, blurred vision. Dermatological reactions: rash, darkening of the skin of the eyelids and local skin reactions on the eyelids. From the nervous system: dizziness, headache. From the respiratory system: bronchial asthma (including acute attacks or exacerbation of the disease in patients with a history of bronchial asthma), shortness of breath. From the musculoskeletal system: muscle pain, joint pain. Other: nonspecific chest pain.
Contraindications
- age under 18 years; - hypersensitivity to latanoprost, benzalkonium chloride or other components of the drug. Use with caution in patients with aphakia, pseudophakia, damage to the posterior capsule of the lens and other risk factors for the development of macular edema (cases of macular edema have been described during treatment with latanoprost , including cystoid), inflammatory, congenital glaucoma due to lack of sufficient experience in using the drug.
Overdose
Symptoms: irritation of the mucous membrane of the eye, hyperemia of the conjunctiva or episclera. Treatment: symptomatic therapy.
special instructions
Latanoprost may cause a gradual change in eye color by increasing the amount of brown pigment in the iris. This effect is detected predominantly in patients with mixed iris color, for example, blue-brown, gray-brown, green-brown or yellow-brown, which is explained by an increase in the melanin content in the stromal melanocytes of the iris. Typically, brown pigmentation extends concentrically around the pupil to the periphery of the iris, and the entire iris or parts of it may become a more intense brown color. In patients with evenly colored eyes of blue, grey, green or brown, changes in eye color were observed very rarely after two years of use of the drug. The color change is not accompanied by any clinical symptoms or pathological changes. After discontinuation of the drug, no further increase in the amount of brown pigment was observed, however, the color change that has already developed may be irreversible. In the presence of nevi or lentigo on the iris, their changes were not observed under the influence of therapy. Before starting treatment, patients should be informed about the possibility of changes in eye color. In case of intense changes in eye pigmentation, treatment may be discontinued. Treatment of only one eye can lead to permanent heterochromia. Latanoprost can cause gradual changes in eyelashes and vellus hair, such as lengthening, thickening, increased pigmentation, increased thickness, and a change in the direction of eyelash growth. Changes in eyelashes are reversible and disappear after cessation of treatment. The drug contains benzalkonium chloride, which can be absorbed into contact lenses. When using contact lenses, they should be removed before instillation and put back on no earlier than 15-20 minutes after instillation of the drug. The bottle must be closed after each use. Do not touch the tip of the pipette to the eye. Impact on the ability to drive vehicles and operate machinery For patients with who experience transient blurred vision after using eye drops, it is not recommended to drive a car or work with moving machinery until it recovers.
Dispensing conditions in pharmacies
On prescription
Side effects
- Irritation or discomfort of the eyes (burning sensation, “sand” in the eyes, itching and tingling), blepharitis, pain, redness of the conjunctiva.
- Increased pigmentation of the iris, swelling of the eyelids and cornea, conjunctivitis, increase in the number, length and thickness of eyelashes, as well as vellus hair.
- Keratitis, iritis/uveitis, macular edema (including cystoid), blurred vision, change in direction of eyelash growth.
- Darkening of the skin of the eyelids, rash, local skin reactions.
- Dizziness, headache.
- Bronchospasm, shortness of breath.
- Pain in joints and muscles, nonspecific pain in the chest area.
Contraindications
Xalatamax should not be prescribed to patients with hypersensitivity to latanoprost and other components of the drug. Caution is required when prescribing the drug to patients with pseudophakia, aphakia, damage to the posterior capsule of the lens, risk factors for macular edema, and other types of glaucoma (inflammatory, congenital).
During pregnancy, Xalatamax is prescribed only when the benefit to the mother exceeds the potential risk to the fetus. Breastfeeding should be stopped during treatment.
special instructions
Before starting treatment with Xalatamax solution, the patient should be warned about possible hyperpigmentation of the iris and eyelids, abnormal growth of eyelashes and vellus hair. This is especially true for people who are prescribed the drug in only one eye, because the color change is irreversible after discontinuation of the drug.
The drug includes benzalkonium chloride, a preservative that is absorbed by contact lenses. Therefore, before introducing it into the eye, the lenses must be removed and reinserted only 20 minutes after instillation.
Patients who notice transient blurred vision after instillation of the solution should avoid driving vehicles and operating complex machinery.
The bottle should be tightly closed after use. Do not touch the dispenser spout to your eye.
Xalatamax solution should be stored in a dark place at room temperature. Keep away from children.
The shelf life indicated on the packaging is 2 years. The solution in an opened bottle is good for no more than 4 weeks.
Xalatamax®
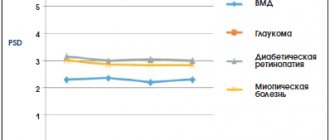
Latanoprost can gradually change eye color by increasing the amount of brown pigment in the iris. Before starting treatment, patients should be informed about the possible permanent change in eye color. Using the drug in one eye may cause irreversible heterochromia. This change in eye color was predominantly observed in patients with unevenly colored irises, namely: brown-blue, gray-brown, yellow-brown and green-brown. In studies of latanoprost, darkening typically began within the first 8 months of treatment, rarely during the second or third year, and was not observed after four years of treatment. The progression of iris pigmentation decreased over time and stabilized after 5 years. There is no data on increased pigmentation over 5 years. In an open-label 5-year safety study of latanoprost, 33% of patients developed iris pigmentation (see section "Side Effects").
In most cases, the change in iris color was minor and often not clinically detected. The incidence ranged from 7 to 85% in patients with unequally colored irises, predominant in patients with yellow-brown irises. No changes were observed in patients with uniformly colored blue irises; in rare cases, changes were observed in uniformly colored gray, green and brown irises.
The change in eye color is caused by an increase in the melanin content in the stromal melanocytes of the iris, and not by an increase in the number of melanocytes themselves. In typical cases, brown pigmentation appears around the pupil and extends concentrically to the periphery of the iris. In this case, the entire iris or parts of it acquire a brown color. After discontinuation of therapy, no further pigmentation was observed. According to available clinical data, the color change was not associated with any symptoms or pathological disorders.
The drug has no effect on nevi and lentigines of the iris.
According to the results of 5-year clinical studies, pigment accumulation in the sclero-corneal trabecular meshwork or other parts of the anterior chamber of the eye was not noted. It has been shown that darkening of the iris does not lead to undesirable clinical consequences, so the use of latanoprost when such darkening occurs can be continued. However, such patients should be monitored regularly and, depending on the clinical situation, treatment may be discontinued.
Experience with the use of latanoprost in the treatment of angle-closure and congenital glaucoma, pigmentary glaucoma, and open-angle glaucoma in patients with pseudophakia is limited.
There is no information on the use of latanoprost in the treatment of secondary glaucoma due to inflammatory eye diseases and neovascular glaucoma.
Latanoprost has no effect on pupil size.
Due to the lack of experience with the use of latanoprost in the treatment of acute attacks of angle-closure glaucoma, the drug should be used with caution in such patients.
Due to the fact that information on the use of latanoprost in the postoperative period of cataract extraction is limited, caution should be exercised when using the drug in this category of patients.
Caution should be exercised when using latanoprost in patients with a history of herpetic keratitis. In case of acute herpetic keratitis, as well as in the case of anamnestic information about chronic recurrent herpetic keratitis, it is necessary to avoid prescribing latanoprost.
Macular edema, including cystoid edema, was observed during latanoprost therapy, mainly in patients with aphakia, pseudophakia, rupture of the posterior lens capsule, or in patients with risk factors for the development of cystoid macular edema (in particular, diabetic retinopathy and retinal vein occlusion). Caution should be exercised when using latanoprost in patients with aphakia, pseudophakia with a posterior capsule tear or anterior chamber intraocular lens, or patients with known risk factors for cystoid macular edema.
Caution should be exercised when using latanoprost in patients with risk factors for developing iritis/uveitis.
Experience with the use of latanoprost in patients with bronchial asthma is limited, but in a number of cases, exacerbation of asthma and/or the appearance of shortness of breath were observed in the post-registration period. Caution should be exercised when using latanoprost in this category of patients (see also section "Side effects").
There have been cases of darkening of the skin of the periorbital area, which in a number of patients was reversible with continued therapy with latanoprost. Latanoprost can cause gradual changes in eyelashes and vellus hair, such as lengthening, thickening, increased pigmentation, increased thickness, and a change in the direction of eyelash growth. Changes in eyelashes were reversible and disappeared after cessation of therapy.
Latanoprost contains benzalkonium chloride, often used as a preservative in ophthalmic medications.
Benzalkonium chloride may cause eye irritation, punctate keratopathy and/or toxic ulcerative keratopathy, and may be absorbed and discolored by soft contact lenses. Careful monitoring of the condition of patients with dry eye syndrome or other corneal diseases is required during long-term use of latanoprost. Before using the drug, you must remove contact lenses and reinsert them no earlier than 15 minutes after instillation (see also section “Method of administration and dosage”).
Children
Information on the effectiveness and safety of latanoprost in children under one year of age is limited. There is no experience with the use of the drug in premature infants (gestational age less than 36 weeks).
There is no information on the safety of long-term use of latanoprost in children. For primary congenital glaucoma in children aged 0 to 3 years, surgical intervention (goniotomy/trabeculotomy) remains the standard treatment method.
Drug interactions
Latanoprost significantly enhances the effect on the level of intraocular pressure of such groups of drugs as: adrenergic agonists, β-blockers, cocarboxylase inhibitors. In addition, it increases to some extent the ability of μ-anticholinergics to reduce intraocular pressure.
Studies conducted outside the human body have shown that the result of mixing this drug with other pharmacological agents containing a substance such as thiomersal is a precipitation reaction. Therefore, to achieve the desired effect, such drugs should be used with a time interval of at least 5 minutes.