Pharmacological properties of the drug Bicyclol
A drug used for liver diseases. The chemical structure is similar to bifendate. The use of Bicyclol helps reduce the increased activity of transaminases in hepatitis, liver damage caused by chloroform, D-galactosamine and paracetamol, and normalize disorders of the structure of the liver tissue of varying degrees of severity. Bicyclol inhibits the production of tumor necrosis factor (TNF-α) by active neutrophils, Kupffer cells and macrophages, and also promotes the removal of free radicals from cells. Thus, Bicyclol inhibits oxidative stress caused by impaired mitochondrial function, which prevents necrosis and apoptosis of hepatocytes. Bicyclol also inhibits hepatocyte apoptosis stimulated by TNF-α and cytotoxic T cells, leading to the restoration of damage to the nucleus and DNA of hepatocytes. Bicyclol also significantly inhibits the expression of HBsAg and HBeAg and significantly reduces the content of hepatitis B virus DNA and hepatitis C virus RNA in the blood in chronic viral hepatitis B and C. The half-life in the alpha phase is 0.84 hours, in the final phase - 6.26 h, the time to reach the maximum concentration in the blood plasma is 1.8 hours, the maximum concentration is 50 ng/ml. The maximum concentration and AUC are directly dependent on the dose of the drug, however, other pharmacokinetic parameters do not change significantly depending on the dose. The drug does not accumulate in the body after repeated administration in normal doses. The maximum serum concentration may increase when taking the drug after meals. Bicyclol metabolism occurs in the liver with the participation of cytochrome P450, with the formation of the main metabolites - 4OH-bicyclol and 4OH-bicyclol. The drug is detected in the blood unchanged 15 minutes after oral administration. The maximum concentration of bicyclol in the liver is achieved 4 hours after taking the drug. The degree of binding to blood plasma proteins reaches 78%. Less than 30% of bicyclol is excreted from the body in feces within 24 hours. About 1.3% of the drug is excreted in the urine, 0.03% in bile.
Bicyclol® (BICYCLOL®)
The active substance of the drug Bicyclol® is a lignan in its chemical structure.
The use of the drug Bicyclol® helps to reduce the increased activity of liver transaminases in hepatitis of various etiologies.
The protective effect of Bicyclol® has been proven in in vitro
and
in vivo
, simulating various farms of liver damage: carbon tetrachloride (CCl4) - damage to biomembranes, necrosis, liver fibrosis, acetaminophen (AP) - glutathione depletion in hepatocytes, D-galactosamine - DNA damage to the hepatocyte nucleus, BCG vaccine and concavalin A (ConA ) - immune damage. Under the influence of Bicyclol®, pathomorphological disorders of the structure of the liver tissue are restored to varying degrees. Bicyclol® inhibits the production of tumor necrosis factor (TNF-α) by active neutrophils, Kupffer cells and macrophages, and also helps to reduce the intensity of free radical processes in cells. Bicyclol® inhibits oxidative activity caused by impaired mitochondrial function, which prevents necrosis and apoptosis of hepatocytes. Bicyclol® also inhibits hepatocyte apoptosis stimulated by TNF-α and cytotoxic T cells, leading to the restoration of damage to the nucleus and DNA of hepatocytes.
In in vivo
in a model of AR liver damage, it was demonstrated that the preliminary administration of bicyclol significantly reduces the degree of damage to hepatocytes, which is expressed in a decrease in the activity of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) - markers of hepatocyte necrosis, in a decrease in the release of cytochrome C and apoptosis-inducing factors from mitochondria, as well as preventing DNA defragmentation.
in vitro studies
It was also found that Bicyclol® is able to suppress the excretion of HBeAg, НBsAg and DNA replication of the hepatitis B virus.
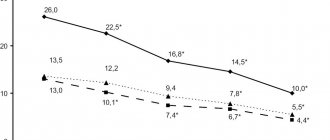
The results of clinical studies of the drug Bicyclol® in patients with chronic viral hepatitis C of moderate activity demonstrated that when it is prescribed at a dose of 150 mg per day for 12 weeks, there is a statistically significant decrease in the biochemical parameters of the inflammatory process in the liver (transaminase activity (ALT, AST ), total, direct and indirect bilirubin), as well as reducing the manifestations of astheno-vegetative syndrome and improving the quality of life of patients.
The data obtained indicate the presence of an anti-inflammatory hepatoprotective effect in the drug Bicyclol® when used as monotherapy in patients with chronic viral hepatitis C. The drug Bicyclol® when prescribed at a dose of 150 mg per day for 12 weeks is characterized by a favorable safety profile and good tolerability .
Use of the drug Bicyclol
Adults and children aged 12 years and older are prescribed 25 mg orally 3 times a day. In some cases, the dose can be increased to 50 mg 3 times a day: in the treatment of chronic hepatitis C; with an uncontrolled increase in transaminase levels or the absence of significant positive dynamics in the levels of ALT and AST during 1–2 months of taking Bicyclol at a dose of 25 mg 3 times a day; for liver cirrhosis, fatty liver, alcoholic hepatitis; while taking nucleoside reverse transcriptase inhibitors (lamivudine). It is advisable to take Bicyclol 2 hours after meals. The minimum duration of treatment is 6 months. If necessary, the course of treatment can be repeated after 1 month.
Bicyclol tablets 25 mg No. 18 - Instructions
Dosage form
Tablets, pack of 18 pieces.
Compound
Active ingredient: bicyclol;
1 tablet contains Bicyclol 25 mg.
Excipients: corn starch, sucrose, sodium starch (type A), magnesium stearate.
Pharmacological group
Drugs used for liver diseases, lipotropic substances.
Pharmacological properties.
Pharmacodynamics. The chemical structure of Bicyclol is similar to bifendate. Data from pharmacodynamic studies have proven that Bicyclol is able to reduce elevated levels of transaminases in hepatitis, liver damage caused by chloroform, D-galactosamine and acetaminophen and restore damage to the structure of liver tissue of varying severity. Due to experimental studies in vitro on a colony of cells 2.2.1.5, it was established that Bicyclol is able to suppress the secretion of hepatitis B virus surface antigen (HBsAg), hepatitis B virus E-antigen (HBeAg), hepatitis B virus DNA and hepatitis C virus RNA. Bicyclol suppresses the production tumor necrosis factor (TNF) by active neutrophils, Kupffer cells and macrophages, and also removes free radicals from cells. Thus, Bicyclol suppresses oxidative stress caused by dysfunction of hepatocyte mitochondria and prevents necrosis and apoptosis in hepatocytes. Bicyclol also delays hepatocyte apoptosis stimulated by tumor necrosis factor and cytotoxic T cells. In turn, this leads to the restoration of damage to the nucleus and DNA of hepatocytes.
Pharmacokinetics. The half-life in the first phase of the two-phase model (t (½) ka) is 0.84 hours, the half-life in the second phase of the two-phase model (t (½) ke) is 6.26 hours, the time to reach maximum concentration (t (peak)) - 1.8 hours, maximum concentration in blood plasma (Cmax) - 50 ng / ml. C max and the area under the concentration-time curve are directly dependent on the dose of the drug taken, but other pharmacokinetic parameters, such as t (½) ka, t (½) ke, Vd / F (volume of distribution ratio (Vd) of the drug means of bioavailability (F), CL / F and t (peak), change insignificantly, depending on the dose, and correspond to the characteristics of linear pharmacokinetics.
The maximum concentration may increase when using the drug after meals.
Bicyclol metabolism occurs in the liver with the participation of cytochrome P450 with the formation of the main metabolites 4 ¢ OH-Bicyclol and 4 OH-Bicyclol.
The drug is observed in human blood unchanged 15 minutes after ingestion. The maximum concentration of Bicyclol is observed in the liver 4:00 after taking the drug. The degree of binding to blood plasma proteins reaches 78%. Less than 30% of Bicyclol is excreted from the body through the digestive tract in feces within 24 hours. Approximately 1.3% is excreted in urine and 0.03% in bile.
Indications for use
Hepatitis accompanied by increased activity of liver transaminases:
- chronic viral hepatitis B;
- chronic viral hepatitis C;
- non-alcoholic steatohepatitis;
- alcoholic hepatitis
- toxic (including drug-induced) hepatitis.
Directions for use and doses
For adults and children over 12 years of age, the drug is prescribed orally at a dose of 25 mg (1 tablet) 3 times a day; if necessary - 50 mg (2 tablets) 3 times a day.
The drug Bicyclol is taken 2:00 after meals.
The minimum treatment period is 6 months or as prescribed by a doctor.
For elderly people (over 70 years old), the dose of the drug is determined individually.
Contraindications
- Hypersensitivity to the components of the drug.
- Acute hepatitis.
- During pregnancy and breastfeeding.
- Age up to 12 years.
Interaction with other drugs and other types of interactions
When Bicyclol is used simultaneously with nucleoside drugs such as lamivudine, the effectiveness of the drug may be reduced.
Features of application
During treatment with Bicyclol, the patient's condition and liver function should be constantly monitored.
Use the drug with caution in patients with hypoalbuminemia, liver cirrhosis, esophageal varices, hepatic encephalopathy, severe hepatitis, renal failure, significantly increased bilirubin levels, ascites, hepatorenal syndrome. The drug should be prescribed with caution for autoimmune hepatitis.
Use during pregnancy or breastfeeding
Do not apply.
The ability to influence the reaction rate when driving vehicles or other mechanisms
Very rarely, dizziness is observed during treatment, so caution should be exercised when driving or operating other machinery.
Children
Do not use in children under 12 years of age.
Overdose
According to clinical studies, when using Bicyclol at a dose of 150 mg 3 times a day, not a single case of overdose was observed. In addition, 400 times the usual human dosage did not cause a toxic reaction.
Adverse reactions
Bicyclol is usually well tolerated. Adverse reactions, if they occur, are temporary, mild or moderate in severity and go away on their own after discontinuation of the drug or with the help of symptomatic therapy. With an incidence of less than 0.5%, dizziness, skin rash, bloating and vomiting may occur. A small number of patients (<0.1%) may experience headache, sleep disturbance, epigastric discomfort, increased transaminase activity, decreased platelet counts, and increased sugar and creatinine levels.
Best before date
3 years.
Storage conditions
In original packaging at a temperature not exceeding 25 ° C
Keep out of the reach of children.
Vacation category
On prescription.
Special instructions for the use of the drug Bicyclol
During the treatment period, the clinical picture and functional state of the liver should be monitored. Bicyclol should be prescribed with caution to patients with decompensated liver function (hypoalbuminemia, liver cirrhosis, esophageal varices, hepatic encephalopathy, severe forms of hepatitis). Should be used with caution in case of autoimmune hepatitis. Use with caution when driving vehicles and working with potentially dangerous mechanisms.