Gliclazide MB, 30 pcs., 60 mg, modified release tablets
Gliclazide is a sulfonylurea derivative that has hypoglycemic properties and is intended for oral administration. Its difference from drugs in this category is the presence of an N-containing heterocyclic ring with an endocyclic bond.
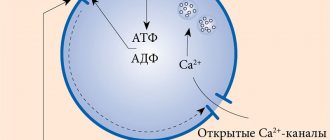
Gliclazide reduces blood glucose levels by stimulating insulin production by beta cells of the islets of Langerhans. Elevated concentrations of C-peptide and postprandial insulin persist after 2 years of treatment. As in the case of other sulfonylurea derivatives, this effect is due to a more intense response of β-cells of the islets of Langerhans to physiological stimulation with glucose. Gliclazide not only affects carbohydrate metabolism, but also provokes hemovascular effects.
In patients with type 2 diabetes mellitus, gliclazide helps restore the early peak of insulin production, which is a consequence of the supply of glucose and stimulates the second phase of insulin secretion. A significant increase in insulin synthesis is associated with a response to stimulation caused by the administration of glucose or food intake.
The use of gliclazide reduces the risk of developing thrombosis of small vessels by influencing mechanisms that can provoke the development of complications in patients with diabetes mellitus [reducing the content of platelet activation factors (thromboxane B2, beta-thromboglobulin), partial inhibition of platelet adhesion and aggregation], as well as influencing the restoration of fibrinolytic activity characteristic of the vascular endothelium, and increased activity of plasminogen, which is a tissue activator. The use of modified-release gliclazide [target glycosylated hemoglobin (HbAlc) is less than 6.5%] with intensive glycemic control in accordance with the results of reliable clinical studies reduces the risk of macro- and microvascular complications accompanying type 2 diabetes compared with traditional glycemic control.
The implementation of intensive glycemic control consists of prescribing gliclazide (average daily dose is 103 mg) and increasing its dose (up to 120 mg per day) when taken against (or instead of) a standard course of therapy before supplementing it with another hypoglycemic drug (for example, insulin, metformin , a thiazolidinedione derivative, an alpha-glucosidase inhibitor). Use of gliclazide in the group of patients undergoing intensive glycemic control (mean HbAlc was 6.5% and mean duration of monitoring 4.8 years) compared with the group of patients undergoing standard control (mean HbAlc was 7.3% ), confirmed that the relative risk of the combined incidence of micro- and macrovascular complications decreases significantly (by 10%) due to a significant reduction in the relative risk of developing major microvascular complications (by 14%), the development and progression of microalbuminuria (by 9%), and the occurrence of complications on the kidneys (by 11%), the occurrence and progression of nephropathy (by 21%), as well as the development of macroalbuminuria (by 30%). When prescribing gliclazide, intensive glycemic control has significant benefits that are not determined by the results of treatment with antihypertensive drugs.
Gliclazide-SZ
Dosage form
extended release tablets
Compound
one tablet contains: active substance, gliclazide - 60 mg excipients: calcium hydrogen phosphate dihydrate - 53.5 mg; maltodextrin - 15.0 mg; hypromellose 4 KM (hydroxypropyl methylcellulose) - 4.3 mg; hypromellose K 100 LV (hydroxypropyl methylcellulose) - 25.7 mg; colloidal silicon dioxide anhydrous (aerosil anhydrous) - 0.5 mg; magnesium stearate - 1.0 mg.
Description
Tablets are white or almost white, round, flat-cylindrical with a chamfer and a score on one side.
ATX code
А10ВВ09
Pharmacological properties
Pharmacodynamics
Gliclazide is a sulfonylurea derivative, a hypoglycemic drug for oral administration, which differs from similar drugs by the presence of an N-containing heterocyclic ring with an endocyclic bond. Gliclazide reduces blood glucose concentrations by stimulating insulin secretion by [3-cells of the islets of Langerhans. Increases in postprandial insulin and C-peptide concentrations persist after 2 years of therapy.
In addition to its effect on carbohydrate metabolism, gliclazide has hemovascular effects.
Effect on insulin secretion In type 2 diabetes mellitus, the drug restores the early peak of insulin secretion in response to glucose and enhances the second phase of insulin secretion. A significant increase in insulin secretion is observed in response to stimulation caused by food intake or glucose administration. Hemovascular effects Gliclazide reduces the risk of thrombosis of small vessels, influencing the mechanisms that can cause the development of complications in diabetes mellitus: partial inhibition of platelet aggregation and adhesion and a decrease in the concentration of platelet activation factors (beta-thromboglobulin, thromboxane Bg), as well as restoration of fibrinolytic activity of the vascular endothelium and increased activity of tissue plasminogen activator.
Intensive glycemic control based on the use of extended-release gliclazide significantly reduces micro- and macrovascular complications of type 2 diabetes mellitus compared with standard glycemic control.
Pharmacokinetics
Absorption After oral administration, gliclazide is completely absorbed. The concentration of gliclazide in the blood plasma increases gradually during the first 6 hours, the plateau level is maintained from 6 to 12 hours. Individual variability is low. Food intake does not affect the rate or extent of absorption of gliclazide.
Distribution Approximately 95% of gliclazide is bound to plasma proteins. The distribution volume is about 30 liters. Taking the drug Gliclazide-SZ at a dose of 60 mg once a day ensures the maintenance of an effective concentration of gliclazide in the blood plasma for more than 24 hours.
Metabolism Gliclazide is metabolized primarily in the liver. There are no active metabolites in blood plasma.
Excretion Gliclazide is excreted mainly by the kidneys: excretion occurs in the form of metabolites, less than 1% is excreted unchanged by the kidneys. The half-life of gliclazide averages from 12 to 20 hours.
Linearity The relationship between the dose taken (up to 120 mg) and the area under the concentration-time pharmacokinetic curve is linear.
Special populations
Aged people
In elderly people, no significant changes in pharmacokinetic parameters are observed.
Indications for use
— Diabetes mellitus type 2 with insufficient effectiveness of diet therapy, physical activity and weight loss. — Prevention of complications of diabetes mellitus: reducing the risk of microvascular (nephropathy, retinopathy) and macrovascular complications (myocardial infarction, stroke) in patients with type 2 diabetes mellitus through intensive glycemic control.
Contraindications
- hypersensitivity to gliclazide, other sulfonylurea derivatives, sulfonamides or excipients included in the drug; — diabetes mellitus type 1; - diabetic ketoacidosis, diabetic precoma, diabetic coma; - severe renal or liver failure (in these cases it is recommended to use insulin); — taking miconazole (see section “Interaction with other drugs”); — pregnancy and breastfeeding (see section “Use during pregnancy and breastfeeding”); - age up to 18 years. It is not recommended to use in combination with phenylbutazone or danazol (see section “Interaction with other drugs”).
Carefully
Old age, irregular and/or unbalanced diet, glucose-6-phosphate dehydrogenase deficiency, severe diseases of the cardiovascular system, hypothyroidism, adrenal or pituitary insufficiency, renal and/or liver failure, long-term therapy with glucocorticosteroids (GCS), alcoholism.
Use during pregnancy and breastfeeding
Pregnancy
There is no experience with the use of gliclazide during pregnancy. Data on the use of other sulfonylureas during pregnancy are limited. In studies on laboratory animals, no teratogenic effects of gliclazide were identified. To reduce the risk of developing congenital defects, optimal control (appropriate therapy) of diabetes mellitus is necessary. Oral hypoglycemic drugs are not used during pregnancy. Insulin is the drug of choice for the treatment of diabetes mellitus in pregnant women. It is recommended to replace the intake of oral hypoglycemic drugs with insulin therapy both in the case of a planned pregnancy and if pregnancy occurs while taking the drug.
Breastfeeding period
Taking into account the lack of data on the passage of gliclazide into breast milk and the risk of neonatal hypoglycemia, breastfeeding is contraindicated during drug therapy.
Directions for use and doses
THE DRUG IS INTENDED FOR TREATMENT OF ADULTS ONLY. The recommended dose of the drug should be taken orally, 1 time per day, preferably during breakfast. The daily dose can be 30 - 120 mg (% - 2 tablets) in one dose. It is recommended to swallow the tablet or half of the tablet whole, without chewing or crushing. If you miss one or more doses of the drug, you cannot take a higher dose at the next dose; the missed dose should be taken the next day. As with other hypoglycemic drugs, the dose of the drug in each case must be selected individually, depending on the concentration of blood glucose and glycated hemoglobin (HbAlc).
Initial dose
The initial recommended dose (including for elderly patients, >65 years) is 30 mg per day ('/g tablet). If adequately controlled, the drug at this dose can be used for maintenance therapy. In case of inadequate glycemic control, the daily dose of the drug can be sequentially increased to 60, 90 or 120 mg. An increase in the dose is possible no earlier than after 1 month of drug therapy at the previously prescribed dose. The exception is patients whose blood glucose concentrations have not decreased after 2 weeks of therapy. In such cases, the dose of the drug can be increased 2 weeks after starting treatment. The maximum recommended daily dose of the drug is 120 mg. The presence of a risk on 60 mg tablets allows you to divide the tablet and take a daily dose of both 30 mg (V2 tablets 60 mg) and, if necessary, 90 mg (1 tablet 60 mg and */2 tablets 60 mg).
Switching from taking another hypoglycemic drug to Gliclazide-SZ, extended-release tablets 60 mg
Gliclazide-SZ, extended-release tablets 60 mg can be used instead of another oral hypoglycemic drug. When transferring patients receiving other oral hypoglycemic drugs to Gliclazide-SZ, their dose and half-life should be taken into account. As a rule, no transition period is required. The initial dose should be 30 mg and then titrated according to blood glucose concentrations. When replacing sulfonylurea derivatives with a long half-life with Gliclazide-SZ, to avoid hypoglycemia caused by the additive effect of two hypoglycemic agents, you can stop taking them for several days. The initial dose of Gliclazide-SZ is also 30 mg (*/g tablet 60 mg) and, if necessary, can be increased further, as described above.
Combined use with another hypoglycemic drug
The drug Gliclazide-SZ can be used in combination with biguanidines, alpha-glucosidase inhibitors or insulin. If glycemic control is inadequate, additional insulin therapy should be prescribed with close medical supervision.
Prevention of complications of diabetes mellitus
To achieve intensive glycemic control, the dose of Gliclazide-SZ can be gradually increased to 120 mg/day in addition to diet and exercise until the target HbAlc level is achieved. The risk of hypoglycemia should be remembered. In addition, other hypoglycemic drugs, such as metformin, an alpha-alpha glucosidase inhibitor, a thiazolidinedione derivative, or insulin, can be added to therapy. Elderly patients
No dose adjustment is required for patients over 65 years of age.
Patients with kidney failure
No dose adjustment is required in patients with mild to moderate renal failure.
Close medical monitoring is recommended Patients at risk of developing hypoglycemia
In patients at risk of developing hypoglycemia (insufficient or unbalanced nutrition; severe or poorly compensated endocrine disorders - pituitary and adrenal insufficiency, hypothyroidism; withdrawal of glucocorticosteroids (GCS) after their long-term use and/or use in high doses ; severe diseases of the cardiovascular system - severe coronary heart disease, severe atherosclerosis of the carotid arteries, widespread atherosclerosis) it is recommended to use a minimum dose (30 mg) of the drug Gliclazide-SZ.
Children and adolescents under 18 years of age.
There are no data on the effectiveness and safety of the drug in children and adolescents under 18 years of age.
Side effect
Given the experience with the use of gliclazide, you should be aware of the possibility of developing the following side effects.
Hypoglycemia
Like other sulfonylurea drugs, Gliclazide-SZ can cause hypoglycemia if meals are not taken regularly and especially if meals are missed. Possible symptoms of hypoglycemia: headache, severe hunger, nausea, vomiting, fatigue, sleep disturbance, irritability, agitation, decreased concentration, slow reaction, depression, confusion, blurred vision and speech, aphasia, tremors, paresis, loss of self-control , feeling of helplessness, impaired perception, dizziness, weakness, convulsions, bradycardia, delirium, shallow breathing, drowsiness, loss of consciousness with the possible development of coma, even death. Andrenergic reactions may also occur: increased sweating, clammy skin, anxiety, tachycardia, increased blood pressure, palpitations, arrhythmia and angina. As a rule, the symptoms of hypoglycemia are relieved by taking carbohydrates (sugar). Taking sweeteners is ineffective. Against the background of other sulfonylurea derivatives, relapses of hypoglycemia were observed after its successful relief. For severe or prolonged hypoglycemia, emergency medical attention, possibly hospitalization, is indicated, even if carbohydrate intake is effective.
Other side effects
From the gastrointestinal tract:
abdominal pain, nausea, vomiting, diarrhea, constipation.
Taking the drug during breakfast can avoid or minimize these symptoms. The following side effects are less common: From the skin and subcutaneous tissue:
rash, itching, urticaria, Quincke's edema, erythema, maculopapular rash, bullous reactions (such as Stevens-Jones syndrome and toxic epidermal necrolysis).
From the hematopoietic organs and lymphatic system:
hematological disorders (anemia, leukopenia, thrombocytopenia, granulocytopenia) rarely develop.
As a rule, these phenomena are reversible if therapy is discontinued. From the liver and biliary tract:
increased activity of liver enzymes (aspartate aminotransferase (ACT), alanine aminotransferase (ALT), alkaline phosphatase), hepatitis (isolated cases).
If cholestatic jaundice appears, therapy should be discontinued. These effects are usually reversible if therapy is discontinued. On the part of the organ of vision:
transient visual disturbances may occur caused by changes in blood glucose concentrations, especially at the beginning of therapy.
Side effects inherent in sulfonylurea derivatives:
as with the use of other sulfonylurea derivatives, the following side effects were noted: erythrocytopenia, agranulocytosis, hemolytic anemia, pancytopenia, allergic vasculitis, hyponatremia. There was an increase in the activity of liver enzymes, impaired liver function (for example, with the development of cholestasis and jaundice) and hepatitis; manifestations decreased over time after discontinuation of sulfonylurea drugs, but in some cases led to life-threatening liver failure.
Overdose
In case of overdose with sulfonylurea derivatives, hypoglycemia may develop. If moderate symptoms of hypoglycemia occur without impairment of consciousness or neurological symptoms, increase the intake of carbohydrates from food, reduce the dose of the drug and/or change the diet. Close medical monitoring of the patient's condition should continue until it is certain that nothing threatens his health. Severe hypoglycemic conditions may develop, accompanied by coma, seizures or other neurological disorders. If such symptoms appear, emergency medical care and immediate hospitalization are necessary. In case of hypoglycemic coma or if it is suspected, the patient is injected intravenously with 50 ml of a 20-40% dextrose (glucose) solution. Then a 5-10% dextrose solution is administered intravenously to maintain the blood glucose concentration above 1 g/l. Careful monitoring of blood glucose levels and observation of the patient should be carried out for at least the next 48 hours. After this period of time, depending on the patient’s condition, the attending physician decides on the need for further observation. Dialysis is ineffective due to the pronounced binding of gliclazide to plasma proteins.
Interaction with other drugs
1) Drugs and substances that increase the risk of developing
hypoglycemia:
(increasing the effect of gliclazide)
Contraindicated combinations
-
Miconazole
(with systemic administration and when using the gel on the oral mucosa): enhances the hypoglycemic effect of gliclazide (possible development of hypoglycemia up to the state of coma).
Not recommended combinations
-
Phenylbutazone
(systemic administration): enhances the hypoglycemic effect of sulfonylurea derivatives (displaces them from their association with plasma proteins and/or slows down their elimination from the body).
It is preferable to use another anti-inflammatory drug. If taking phenylbutazone is necessary, the patient should be warned about the need for glycemic control. If necessary, the dose of Gliclazide-SZ should be adjusted while taking phenylbutazone and after its termination. — Ethanol
: enhances hypoglycemia, inhibiting compensatory reactions, can contribute to the development of hypoglycemic coma.
It is necessary to stop taking medications that contain ethanol and drinking alcohol. Combinations requiring precautions
Taking gliclazide in combination with certain medications: other hypoglycemic agents (insulin, acarbose, metformin, thiazolidinidones, dipeptidyl peptidase-4 inhibitors, GLP-1 agonists);
beta-blockers, fluconazole; angiotensin-converting enzyme inhibitors - captopril, enalapril; Hg-histamine receptor blockers; monoamine oxidase inhibitors; sulfonamides; clarithromycin and non-steroidal anti-inflammatory drugs) is accompanied by an increased hypoglycemic effect and the risk of hypoglycemia. 2) Drugs that increase blood glucose levels:
(weakening the effect of gliclazide)
Not recommended combinations
-
Danazol:
has a diabetogenic effect.
If taking this drug is necessary, the patient is advised to carefully monitor blood glucose. If it is necessary to take drugs together, it is recommended to select the dose of a hypoglycemic agent both while taking danazol and after its discontinuation. Combinations requiring precautions
-
Chlorpromazine (antipsychotic):
in high doses (more than 100 mg per day) increases the concentration of blood glucose, reducing insulin secretion.
Careful glycemic control is recommended. If it is necessary to take drugs together, it is recommended to select the dose of a hypoglycemic agent, both while taking the antipsychotic and after its withdrawal. - GCS
(systemic and local use: intra-articular, skin, rectal administration) and
tetracosactide
: increase the concentration of blood glucose with the possible development of ketoacidosis (decreased tolerance to carbohydrates).
Careful glycemic control is recommended, especially at the beginning of treatment. If it is necessary to take drugs together, it may be necessary to adjust the dose of the hypoglycemic agent both while taking GCS and after their withdrawal. - Ritodrine, salbutamol, terbutaline
(intravenous administration): beta-2 adrenergic agonists help increase blood glucose concentrations.
Particular attention should be paid to the importance of self-glycemic control. If necessary, it is recommended to transfer the patient to insulin therapy. 3) Combinations that should be taken into account
-
Anticoagulants
(eg, warfarin) Sulfonylureas may enhance the effect of anticoagulants when taken together. Anticoagulant dose adjustment may be required.
special instructions
Hypoglycemia
When taking sulfonylurea derivatives, including gliclazide, hypoglycemia may develop, in some cases in a severe and prolonged form, requiring hospitalization and intravenous administration of dextrose solution for several days (see section “Side Effects”).
The drug can be prescribed only to those patients whose meals are regular and include breakfast. It is very important to maintain a sufficient intake of carbohydrates from food, as the risk of developing hypoglycemia increases with irregular or insufficient nutrition, as well as when consuming foods low in carbohydrates. Hypoglycemia is more likely to occur during a low-calorie diet, after prolonged or vigorous exercise, after drinking alcohol, or when taking multiple hypoglycemic medications at the same time. Typically, symptoms of hypoglycemia go away after eating a meal rich in carbohydrates (such as sugar). It should be borne in mind that taking sweeteners does not help eliminate hypoglycemic symptoms. Experience with other sulfonylureas suggests that hypoglycemia may recur despite initial effective management of the condition. If hypoglycemic symptoms are pronounced or prolonged, even if the condition improves temporarily after eating a meal rich in carbohydrates, emergency medical care is necessary, including hospitalization. To avoid the development of hypoglycemia, careful individual selection of drugs and dosage regimen is necessary, as well as providing the patient with complete information about the treatment being carried out. An increased risk of developing hypoglycemia may be observed in the following cases: - refusal or inability of the patient (especially the elderly) to follow the doctor’s orders and control their condition; - insufficient and irregular nutrition, skipping meals, fasting and changes in diet; — imbalance between physical activity and the amount of carbohydrates taken; - renal failure; - severe liver failure; — overdose of the drug Gliclazide-SZ; - some endocrine disorders: thyroid diseases, pituitary and adrenal insufficiency; - simultaneous use of certain medications (see section “Interaction with other medications”). Renal and hepatic impairment
In patients with hepatic and/or severe renal impairment, the pharmacokinetic and/or pharmacodynamic properties of gliclazide may be altered.
The state of hypoglycemia that develops in such patients can be quite long-lasting; in such cases, immediate appropriate therapy is necessary. Inadequate glycemic control
Glycemic control in patients receiving hypoglycemic agent therapy may be impaired in the following situations: fever, injury, infectious diseases, or major surgery.
In these conditions, it may be necessary to stop therapy with Gliclazide-SZ and prescribe insulin therapy. In many patients, the effectiveness of oral hypoglycemic agents, including gliclazide, tends to decrease after an extended period of treatment. This effect may be due to both progression of the disease and a decrease in the therapeutic response to the drug. This phenomenon is known as secondary drug resistance, which must be distinguished from primary resistance, in which the drug does not give the expected clinical effect even at the first prescription. Before diagnosing secondary drug resistance in a patient, it is necessary to assess the adequacy of dose selection and the patient’s compliance with the prescribed diet. Laboratory tests
To assess glycemic control, regular determination of fasting blood glucose and glycated hemoglobin HbAlc is recommended.
In addition, it is advisable to regularly self-monitor blood glucose concentrations. Sulfonylureas may cause hemolytic anemia in patients with glucose-6-phosphate dehydrogenase deficiency. Since gliclazide is a sulfonylurea derivative, caution must be exercised when prescribing it to patients with glucose-6-phosphate dehydrogenase deficiency. The possibility of prescribing a hypoglycemic drug of another group should be assessed. Information for patients
It is necessary to inform the patient, as well as his family members, about the risk of developing hypoglycemia, its symptoms and conditions that contribute to its development. The patient must be informed of the potential risks and benefits of the proposed treatment. The patient must be explained the importance of diet, the need for regular exercise and monitoring blood glucose concentrations.
Impact on the ability to drive vehicles and operate machinery
Due to the possible development of hypoglycemia when using the drug Gliclazide-SZ, patients should be aware of the symptoms of hypoglycemia and should exercise caution when driving or performing work that requires a high speed of physical and mental reactions, especially at the beginning of therapy.
Release form
Extended release tablets, 60 mg. 10 or 30 tablets in blister packs. 30 tablets in a polymer jar or in a polymer bottle. Each jar, bottle or 3, 6 blister packs of 10 tablets or 1, 2, 3 blister packs of 30 tablets along with instructions for use in a cardboard box.
Best before date
3 years. Do not use after the expiration date stated on the package.
Storage conditions
In a place protected from light, at a temperature not exceeding 25 °C. Keep out of the reach of children.
Vacation conditions
Dispensed by prescription.
Prescription drug
Prescription drug