Hydrochloric or hydrochloric acid is a solution of hydrogen chloride (HCl) in water. Strong monobasic volatile acid. A typical representative of mineral (inorganic) acids, it is present in many chemical reactions as a reagent or catalyst, for example in esterification reactions - the formation of an ester from a carboxylic acid and an alcohol.
Secreted in the stomach of humans and other mammals. Let's find out what hydrochloric acid looks like, what it reacts with and where it is used.
Physical properties
Hydrochloric acid is a clear, colorless liquid with a characteristic pungent odor.
Formed when hydrogen chloride is passed through water. It also participates in reactions in the liquid state. It dissolves well in water, i.e. mixes with it. Industrially produced hydrochloric acid is slightly yellow in color due to elemental impurities. Concentrated acid, like hydrogen chloride, “smoke” in moist air. A molecular crystal lattice, in the nodes of which there are HCl molecules, in the molecule there is a covalent polar bond.
Receipt
Hydrochloric acid is prepared by dissolving hydrogen chloride gas in water.
Hydrogen chloride is produced by burning hydrogen in chlorine; the acid obtained in this way is called synthetic. Hydrochloric acid is also obtained from exhaust gases - by-product gases formed during various processes, for example, during the chlorination of hydrocarbons. The hydrogen chloride contained in these gases is called free gas, and the acid thus obtained is called free gas. In recent decades, the share of gas-free hydrochloric acid in production volume has gradually increased, displacing acid produced by burning hydrogen in chlorine. But hydrochloric acid obtained by burning hydrogen in chlorine contains fewer impurities and is used when high purity is required. In laboratory conditions, a method developed by alchemists is used, which consists of the action of concentrated sulfuric acid on table salt:
NaCl + H2SO4 →150oC NaHSO4 + HCl
At temperatures above 550 °C and excess table salt, interaction is possible:
2NaCl + H2SO4 →550oC Na2SO4 + 2HCl
It is possible to obtain by hydrolysis of magnesium and aluminum chlorides (hydrated salt is heated):
MgCl2 ⋅ 6H2O →t.oC MgO + 2HCl + 5H2O AlCl3 ⋅ 6H2O →t.oC Al(OH)3 + 3HCl + 3H2O
These reactions may not proceed to completion with the formation of basic chlorides (oxychlorides) of variable composition, for example:
MgCl2 + H2O → Mg2OCl2 + HCl
Hydrogen chloride is highly soluble in water. Thus, at 0 °C, 1 volume of water can absorb 507 volumes of HCl, which corresponds to an acid concentration of 45%. However, at room temperature the solubility of HCl is lower, so in practice 36% hydrochloric acid is usually used.
Chemical properties of hydrochloric acid
Being a monobasic acid, i.e. it contains one hydrogen atom, it dissociates in one step:
HCl = H+ + Cl-
The solution has an acidic environment, the pH of the solution is 1-2 depending on the concentration of ions. Litmus turns red and methyl orange turns pink. Therefore, hydrochloric acid exhibits acidic properties and reacts with basic compounds. Due to the presence of chlorine, it is a fairly strong oxidizing agent. In this case, Cl has a valency of 1 and an oxidation state of -1.
- Interaction with active metals (in the voltage series up to hydrogen). An acid salt is formed and molecular hydrogen is released:
- 2K + 2HCl → 2KCl + H2 Salts of hydrochloric acid are called chlorides.
- With oxides of all metals with the formation of salt and the release of water:
- ZnO + 2HCl → H2O + ZnCl2
- With hydroxides (bases) of all metals:
- Ca(OH)2 + 2HCl → CaCl2 + H2O This is a neutralization reaction. The products contain chloride and water. It does not form acidic or basic salts because it is monobasic.
- Displacement of weaker volatile acids from metal salts:
- Na2CO3 + 2 HCl → H2O + CO2 + 2 NaCl Usually, during such reactions a gas is released, since most weak acids are very volatile, or a precipitate forms, for example in the case of silicic acid H2SiO3, which is not soluble in water.
- Na2CO3 + 2 HCl → H2O + CO2 + 2 NaCl Usually, during such reactions a gas is released, since most weak acids are very volatile, or a precipitate forms, for example in the case of silicic acid H2SiO3, which is not soluble in water.
- Interaction with strong oxidizing agents (chromates, dichromates, KMnO4):
- 2 KMnO4 + 16 HCl → 8 H2O + 5 Cl2 + 2 KCl + 2 MnCl2 This is a redox reaction. The only case when hydrochloric acid is a reducing agent and not an oxidizing agent.
- Specific reaction with ammonia:
- NH3 + HCl → NH4 Cl By nature, this is a neutralization reaction, where hydrochloric acid is an oxidizing agent, and ammonia acts as a hydroxide (basic in nature). The salt formed in this reaction is released in the form of a thick white vapor with small crystals of ammonium chloride.
Qualitative reaction to chloride ion: reaction with soluble silver salts (acetate or nitrate): AgNO3 + HCl → HNO3 + AgCl. A white cheesy precipitate of AgCl forms, insoluble in all acids except sulfuric acid, as well as in excess ammonia.
Features of treatment
Highly concentrated hydrochloric acid is a caustic substance
, upon contact with skin causes severe chemical burns. Contact with eyes is especially dangerous. To neutralize burns, use a solution of a weak base, or a salt of a weak acid, usually baking soda.
When opening vessels with concentrated hydrochloric acid, hydrogen chloride vapors, attracting air moisture, form a fog that irritates the eyes and respiratory tract of humans.
Reacting with strong oxidizing agents (bleach, manganese dioxide, potassium permanganate) forms toxic chlorine gas.
In the Russian Federation, the circulation of hydrochloric acid with a concentration of 15% or more is limited.
Application of acid
Since hydrochloric acid is a caustic toxic substance, its turnover should not exceed 15%, and when conducting experiments it is necessary to use gloves and ventilation. Finds application in several industries:
- In metallurgy it is used for cleaning and disinfecting the surface of metals during soldering and for producing chlorides of zinc, iron and other metals;
- Acidity regulator E507;
- In medicine it is used to normalize the functioning of the stomach in case of dyspepsia. Available in tablets mixed with pepsin (a proteolytic enzyme), it activates its action when the drug dissolves. This solution has a pH of 3-5.
Synonym: hydrochloric acid.
Properties.
Colorless, transparent, volatile liquid with a peculiar odor and sour taste. Miscible with water and alcohol in all respects, forming acidic solutions. Specific gravity 1.125-1.127.
Store according to list B, in bottles with ground-in stoppers.
In practice there are: a) hydrochloric acid containing 25% hydrogen chloride b) strong reactive hydrochloric acid containing 35-37% hydrogen chloride c) diluted hydrochloric acid - a transparent colorless liquid, miscible with water in all proportions. Prepared by mixing 1 part hydrochloric acid with 2 parts water. Contains 8.2-8.4% hydrogen chloride. For medicinal purposes, diluted hydrochloric acid is used and prescribed in recipes.
Action and application.
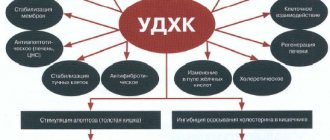
Changes pepsinogen into active pepsin, providing an acidic environment for its action. In the stomach, it promotes the digestion of proteins, creates conditions for the evacuation of contents into the intestines, regulates the tone of the pylorus, enhances the secretion of the pancreas and bile, has an antimicrobial effect, prevents the development of putrefactive and fermentative processes and prevents the penetration of pathogenic bacteria into the intestines. Has a strong bactericidal effect. As the temperature of the solvent increases, the disinfecting power increases.
Kills spore and vegetative forms of microbes. In the presence of small quantities of sodium chloride, the bactericidal power of the acid increases, since it increases the permeability of the acid into the thickness of the skin, and in large quantities, its activity decreases; 2% hydrochloric acid in the presence of 10% sodium chloride kills anthrax spores in raw hides at a temperature of 40°C for nine hours.
Used internally for low acidity of gastric juice, fermentation and putrefactive processes in the stomach, alkali poisoning, indigestion with symptoms of dyspepsia. In particular, it is prescribed for chronic hypo- and anocidal gastritis, chronic gastroenteritis, for hypotension and atony of the rumen, for tympanic rumen, simple and toxic dyspepsia. To accelerate the absorption of iron in anemia. For chronic gastric catarrh, the stomach is washed with an acid solution (0.3%). For inflammation of goiter in birds and cholera in chickens, a 0.4% solution is drunk ad libitum. A 1% hydrochloric acid solution of pepsin (1 liter of distilled water, 5 ml of pure hydrochloric acid and pepsin) is used for dyspepsia of young animals (calves 100 ml; piglets 50 ml, lambs 30 ml 2-3 times a day).
Hydrochloric acid is used to produce artificial gastric juice. It is used to disinfect drinking water and hides unsafe for anthrax. To disinfect raw hides, use a 2.5% solution based on hydrogen chloride with the addition of 10% table salt at a temperature of 40°C and an exposure time of 9 hours. Taking into account the absorption of hydrochloric acid by the skins, an excess amount of acid is taken, but not more than 5% of the weight of the skins. The disinfectant solution is used 10 times the weight of the raw hide. Based on this, for 100 kg of raw hides you need to take 1000 liters of a 2% solution of hydrochloric acid (20 liters of acid) and add 5 liters of acid for adsorption by the leather (you will get a 2.5% pickle solution). Hydrochloric acid with sodium hyposulfite, according to the Demyanovich method, is used to treat scabies.
Hydrochloric acid is administered orally in the form of a 0.1-0.4% aqueous solution, preferably with pepsin.
Doses of diluted acid orally:
horses 10-20 ml; cattle 15-30 ml, small cattle 2-5 ml, pigs 1-2 ml, dogs 0.1-0.5 ml, cats 0.1-0.2 ml, chickens 0.1-0.5 ml.
Use in the food industry
The food industry uses E507 in the processing of various products. Its main use in the food industry is in the production of corn syrups, especially high fructose corn syrups. It is also often found in mayonnaise and is part of citric acid, gelatin, and fructose.
Hydrochloric acid can also be used for acid modification of corn starch and for adjusting the pH of intermediates and final products.
The most common use is in the production of soft drinks, which accounts for 70-75% of demand.
E507 is also used in other areas of the food industry, including the production of hydrolyzed vegetable protein and soy sauce. It is used to acidify crushed bones, to produce gelatin, and as an acidifying agent for foods such as sauces, vegetable juices, and canned foods.
Hydrochloric acid is often used in the production of:
- artificial sweeteners;
- lysine and choline chloride (both used primarily as animal feed additives);
- citric acid;
- corn starch;
- soft drinks;
- soy sauce.
How is hydrochloric acid produced in the laboratory?
The production of the substance is large-scale, sale is free. In laboratory experiments, a solution is produced by the action of high concentration sulfuric acid on ordinary kitchen salt (sodium chloride).
There are 2 methods for dissolving hydrogen chloride in water:
- Hydrogen is burned in chlorine (synthetic).
- Associated (absorbed). Its essence is to carry out organic chlorination, dehydrochlorination.
The substance is easily synthesized by pyrolysis of organochlorine waste. This happens as a result of the breakdown of hydrocarbons with a complete lack of oxygen. You can also use metal chlorides, which are raw materials for inorganic substances. If there is no concentrated sulfuric acid (electrolyte), take diluted one.
As for obtaining the reagent in natural conditions, most often this chemical mixture can be found in the waters of volcanic waste. Hydrogen chloride is a component of the minerals sylvite (potassium chloride, similar in appearance to game dice), bischofite. All these are methods of extracting the substance in industry.
In the human body, this enzyme is found in the stomach. A solution can be either an acid or a base. One of the common extraction methods is called sulfate.
Formula and other names of hydrochloric acid
Hydrochloric acid contains two chemical elements: chlorine and hydrogen. This acid consists of two atoms and has the formula: HCl. It is worth noting that hydrochloric acid is a trivial name (i.e., a name used in everyday speech by chemists that does not reflect its composition). According to the international IUPAC nomenclature, a substance with the formula HCl is usually called hydrochloric acid. Sometimes HCl is called hydrochloric acid or hydrogen chloride, or hydrogen chloride is also an acceptable name.
Use in everyday life
HCl is one of the most powerful cleaning agents available today, it is extremely effective and recommended as a cleaner and can be used to clean any product that can withstand its effects.
HCl is used to neutralize the water, making it safe for bathers.
Most often the pH level is high; The best way to lower the pH is to slowly pour muriatic acid directly into the deep end of the pool while the pool pump is on and the water is circulating.
Industrial Applications
Most of the hydrochloric acid consumed in industry is used to regenerate ion exchange resins, which are used to remove impurities. It is used primarily for continuous steel pickling operations, but is also used in aluminum pickling and metal cleaning.
HCl is used to both remove rust, scale, and unwanted carbonate deposits in oil wells to stimulate the flow of crude oil or gas into the well.
Neutralization of hydrochloric acid with limestone (CaCO3) causes the formation of calcium chloride. Calcium chloride is used for dust control, industrial processing, oil production, concrete processing and tire ballasting.
Aqueous hydrochloric acid is used in many different applications. These include recovery of semi-precious metals from spent catalysts, use as a catalyst in synthesis, pH adjustment, regeneration of ion exchange resins used in wastewater and power treatment, neutralization of alkaline products or waste, and brine acidification for use in the production of chlorine and caustic soda .