| Potassium chloride | |
| Are common | |
| Systematic name | potassium chloride |
| Traditional names | potassium chloride |
| Chem. formula | |
| Physical properties | |
| Molar mass | 74.555 g/mol |
| Density | 1.984 g/cm³ |
| Thermal properties | |
| Temperature | |
| • melting | 776 °C |
| • boiling | 1407 °C |
| Mol. heat capacity | 51.30 J/(mol K) |
| Chemical properties | |
| Solubility | |
| • in water at 0 °C | 28.1 g/100 ml |
| • in water at 20 °C | 34.0 g/100 ml |
| • in water at 100 °C | 56.7 g/100 ml |
| Classification | |
| Reg. CAS number | 7447-40-7 |
| PubChem | 4873 |
| Reg. EINECS number | 231-211-8 |
| SMILES | [Cl-].[K+] |
| InChI | InChI=1S/ClH.K/h1H;/q;+1/p-1 WCUXLLCKKVVCTQ-UHFFFAOYSA-M |
| ex Alimentarius | E508 |
| RTECS | TS8050000 |
| ChEBI | 32588 |
| ChemSpider | 4707 |
| Safety | |
| LD50 | 2600 mg/kg |
| Toxicity | low toxicity |
| NFPA 704 | 0 1 0 |
| Data given is based on standard conditions (25 °C, 100 kPa) unless otherwise stated. | |
| Media files on Wikimedia Commons | |
Potassium chloride
(KCl; potassium salt, sylvite) is a chemical compound, an inorganic compound with the composition KCl. It is the average potassium salt of hydrochloric acid.
Forms a white, odorless crystalline substance, highly soluble in water. Belongs to the NaCl structural type. It occurs in nature in the form of the minerals sylvite and carnallite, and is also part of sylvinite.
Brief characteristics of potassium chloride:
Potassium chloride is a white inorganic substance.
The chemical formula of potassium chloride is KCl.
Potassium chloride is an inorganic chemical compound, a salt of hydrochloric (hydrochloric) acid and potassium, a binary compound of potassium and chlorine.
It dissolves well in water. Practically insoluble in acetone, ethanol, methanol.
Does not form crystalline hydrates.
Non-flammable, fire and explosion proof.
Potassium chloride, in terms of the degree of impact on the body, belongs to the 3rd hazard class according to GOST 12.1.007 as a moderately hazardous substance. Does not form toxic compounds in the air.
Not corrosive.
Potassium chloride is a food additive E508.
Potassium chloride occurs naturally in the form of the minerals sylvite and carnallite, and is also part of sylvinite.
Application
- Potassium chloride is used for the production of KOH, KCO3, KClO4, KNO3 and other potassium compounds.
- In agriculture, potassium chloride is a common potash fertilizer.
- In the food industry, sodium chloride is used as an additive (E508) to table salt (“reduced sodium salt”).
- In medicine, solutions of potassium chloride are used orally or intravenously in cases of potassium deficiency in the body (for example, during treatment with diuretics, prolonged vomiting), in case of heart rhythm disturbances, etc.
Potassium ions play a very important role in regulating body functions. Potassium salts are quickly excreted by the kidneys. Like other potassium salts, potassium chloride has a moderate diuretic effect.
Physical properties of potassium chloride:
| Parameter name: | Meaning: |
| Chemical formula | KCl |
| Synonyms and names in a foreign language | potassium chloride (English) potassium chloride (Russian) |
| Type of substance | inorganic |
| Appearance | colorless cubic crystals |
| Color | white, colorless |
| Taste | salty |
| Smell | without smell |
| Physical state (at 20 °C and atmospheric pressure 1 atm.) | solid |
| Density (state of matter – solid, at 20 °C), kg/m3 | 1984 |
| Density (state of matter – solid, at 20 °C), g/cm3 | 1,984 |
| Boiling point, °C | 1407 |
| Melting point, °C | 776 |
| Molar mass, g/mol | 74,555 |
| Solubility in water (20 oС), g/100 g | 34,4 |
Notes[ | ]
- Vladimirov D. A. et al.
Optimization of recording holograms on additively colored KCl crystals (inaccessible link) // Optics and Spectroscopy. - 2005. - T. 99, No. 1. - P. 147-150. (unavailable link) - In Washington, the composition of the lethal injection was changed (unspecified)
. Lenta.ru (March 3, 2010). Access date: August 14, 2010. - V. V. Kovzov, I. V. Fomchenko, M. A. Makaruk, V. A. Yurkevich.
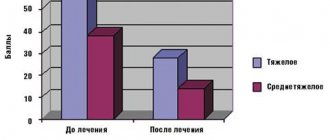
Evaluation of the effectiveness of the use of the drugs "Vethydron" and "Rehydravet" in the complex treatment of piglets and calves with gastrointestinal diseases (other). - 2012. - ISSN 2078-0109.
Preparation of potassium chloride:
In industry, potassium chloride is obtained from the natural mineral sylvinite using the methods of halurgy and flotation. Moreover, the halurgical method for producing potassium chloride is based on the different solubility of KCl and NaCl in water at elevated temperatures.
Potassium chloride is obtained as a result of the following chemical reactions:
- 1. interaction of potassium, potassium oxide and potassium hydroxide with hydrochloric acid.
- 2. interactions between potassium and chlorine:
2K + Cl2 → 2KCl.
- 3. interaction of potassium hydroxide and aluminum chloride:
AlCl3 + 3KOH → Al(OH)3 + 3KCl.
- 4. interactions between potassium iodide and lead chloride:
AlCl3 + 3KOH → Al(OH)3 + 3KCl.
Potassium chloride: both medicine and poison
A chemical substance with dozens of forms – it’s all about potassium chloride. Doctors have discovered its amazing ability to heal the human body from many ailments, agricultural workers have been using potassium chloride as a mineral fertilizer for decades, and candle makers know very well how to use potassium chloride to give an unusual shade to the flame of a decorative candle.
Potassium chloride : general characteristics
The second name for potassium chloride is potassium salt of hydrochloric acid. The compound is a substance with a white crystalline structure and is odorless. In nature, potassium chloride occurs in the form of minerals:
- Silvin. The mineral was first discovered by Italian geologists who found it on the legendary volcano Vesuvius back in 1832)
Carnallite. This mineral is a double salt - potassium and magnesium chloride. It was named after the discoverer of the mineral, the German Carnall.
Potassium chloride : methods of production
The most common method for producing potassium chloride is the reaction of hydrochloric acid and potassium hydroxide.
Potassium chloride is also successfully extracted from sylvinite. And this is done in two main ways:
- Galurgical method . At low temperature, a saturated solution of potassium chloride and sodium chloride is prepared. Afterwards, the solution is heated and the resulting mixture is treated with sylvinite. During the processing of the mineral, the solution is additionally enriched with potassium chloride, and sodium chloride precipitates and is separated by filtration.
- Flotation method . This method involves separating minerals based on their ability to adhere to the liquid interface between two phases.
Potassium chloride : areas of application
- Agriculture . Agronomists are well aware of the ability of potassium chloride to perfectly fertilize the soil, enriching it with potassium, which is so necessary for normal plant growth.
- Medicine . On pharmacy shelves, potassium chloride can be found in the form of 4 and 10 percent solutions based on glucose solution. Potassium chloride is used for diseases: hypokalemia (lack of potassium in the human body), cardiac arrhythmia, intoxication of the body with certain groups of drugs.
- Making candles . Do you want to create a candle with your own hands with a flame of an unusual color? – Potassium chloride powder is perfect for these purposes. This component is capable of giving the candle flame a noble, purple color. It is enough just to add 1 teaspoon of the substance to the solution for soaking the wick...
There is also perhaps the strangest way to use potassium chloride. Some American states still use the substance... as a means of executing the death penalty in prisons. A prisoner sentenced to death is given a lethal injection of potassium chloride.
Potassium chloride
and candle making
Decorative candle "Royal purple". To create an unusual candle craft we will need:
First, let's make a solution for soaking the wick. To do this, dilute 2 tbsp in half a glass of warm water. salt, 4 tbsp. l. borax and 1 tsp. potassium chloride. Mix the solution thoroughly until all components are completely dissolved. Place the wick in the prepared solution for 30 minutes. Dry the wick in a dry room for several days.
Now you can proceed directly to making the candle. Melt the wax in a water bath and add red dye to the melted candle mass. Stir the wax mixture again until completely homogeneous.
Using a holder, place the soaked and dried wick into the candle mold and fill the mold with melted candle mass. If desired, we decorate the candle with decor. This can be a picture in the style of decoupage, or an unusual relief image using the embossing technique, or ordinary sparkles.
Now that the candle has frozen, you can light it. By adding potassium chloride to the wick solution, the candle flame will turn a beautiful purple color. The substance in a decorative candle is absolutely harmless to human health! By the way, other substances can also give a candle flame an unusual color: lithium chloride, magnesium sulfate or copper sulfate.
Potassium chloride : where to buy
The substance is sold in pharmacies in the form of glucose solutions. The smallest bottle of 10 ml costs only 0.5 USD.
As for potassium chloride as a mineral fertilizer, it costs about 0.5 USD. for 1 kg. If we take potassium chloride as a substance in its pure form, then for one kg manufacturers ask about 0.8-1 USD. You can buy the substance without leaving your home, via the Internet.
Save article:
makecandles.ru
Chemical properties of potassium chloride. Chemical reactions of potassium chloride:
The chemical properties of potassium chloride are similar to those of other metal chlorides. Therefore, it is characterized by the following chemical reactions:
1. reaction between potassium chloride and sodium:
KCl + Na → K + NaCl (t = 760-890 °C).
As a result of the reaction, potassium and sodium chloride are formed. During the reaction, sodium gas is exposed to the potassium chloride melt.
2. reaction between potassium chloride and sodium nitrate:
NaNO3 + KCl → NaCl + KNO3.
As a result of the reaction, potassium nitrate and sodium chloride are formed.
3. reaction between potassium chloride and sodium nitrite:
NaNO2 + KCl → KNO2 + NaCl.
As a result of the reaction, potassium nitrite and sodium chloride are formed.
4. reaction between potassium chloride and silver nitrate:
AgNO3 + KCl → AgCl + KNO3.
As a result of the reaction, potassium nitrate and silver chloride are formed. The reaction uses a dilute solution of potassium chloride.
5. reaction between potassium chloride and zinc chloride:
ZnCl2 + 2KCl → K2[ZnCl4].
As a result of the reaction, potassium tetrachlorozincate is formed.
6. reaction between potassium chloride and aluminum chloride:
KCl + AlCl3 → K[AlCl4].
As a result of the reaction, potassium tetrachloroaluminate is formed.
7. reaction between potassium chloride and palladium chloride:
PdCl2 + 2KCl → K2[PdCl4].
As a result of the reaction, potassium tetrachloropaladate is formed. The reaction uses a concentrated solution of potassium chloride.
8. reaction between potassium chloride and platinum chloride:
PtCl4 + 2KCl → K2[PtCl6].
As a result of the reaction, potassium hexachloroplatinate is formed. The reaction uses a concentrated solution of potassium chloride.
9. reaction between potassium chloride, ferric chloride and water:
FeCl3 + 2KCl + H2O → K2[Fe(H2O)Cl5].
As a result of the reaction, potassium pentachloroaquaferrate is formed. The reaction uses saturated solutions of potassium chloride and ferric chloride.
10. reaction of potassium chloride with mineral acids:
Potassium chloride reacts with mineral acids.
11. electrolysis reaction of an aqueous solution of potassium chloride:
KCl + 3H2O ± 6e– → 3H2 + KClO3 (t = 760-890 °C),
2KCl + 2H2O ± 2е– → H2 + Cl2 + 2KOH.
As a result of the first reaction, hydrogen and potassium chlorate are formed, as a result of the second - hydrogen, chlorine and potassium hydroxide.
12. electrolysis reaction of molten potassium chloride:
2KCl ± 2е– → 2K + Cl2.
As a result of the reaction, hydrogen and chlorine are formed.
Application in veterinary medicine[ | ]
It is used as a component of powder preparations to restore water and electrolyte balance in animals, and is used in complex therapy for diseases associated with diarrheal syndrome (dyspepsia, abomazoenteritis, gastroenteritis, enterocolitis, etc.). 1 g of the drug contains: sodium chloride – 180 mg; sodium citrate – 150 mg; potassium chloride – 140 mg; excipients: glycine, L-isoleucine, lactulose and lactose filler. The powder is dissolved in water and fed to the animal.
Example of drug packaging
Drugs of this type are well absorbed, quickly restore the level of sodium and potassium in the blood plasma, replenish fluid deficiency in the body of animals, regulate acid-base balance, reduce the aggregation of formed elements and blood viscosity, and have detoxifying properties. The components of the drug are natural metabolic metabolites. Sodium and potassium ions restore the functioning of cell membranes in the intestines, myocardium and skeletal muscles. Sodium citrate prevents the development of acidosis. They are used to restore the water-electrolyte balance in the treatment of animals with diseases associated with diarrhea syndrome, it has a high therapeutic effectiveness, which amounted to 91.1% in the complex treatment of weaning piglets with gastrointestinal diseases and in the complex treatment of calves of the colostrum-milk period and period rearing with diseases of the digestive system - 90%. The drugs help normalize blood counts and improve the safety of piglets and calves.[3]