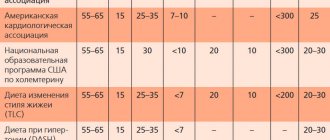
Vilprafen, 1 piece, 15 g, 250 mg/5 ml, granules for the preparation of suspension for oral administration
The use of the following drugs in combination with josamycin is contraindicated due to the potential for serious side effects:
— Ergotamine, dihydroergotamine
The result of the interaction is the risk of severe vasoconstriction (ergotism) with the possible development of limb necrosis (due to inhibition of hepatic metabolism and elimination of ergot alkaloids).
— Cisapride, pimozide
As a result of the interaction, the risk of developing life-threatening arrhythmias, including ventricular tachycardia of the “pirouette” type, increases.
— Ivabradin
As a result of the interaction, the plasma concentration of ivabradine and associated side effects increase (due to inhibition of the hepatic metabolism of ivabradine).
— Colchicine
The interaction results in an increased risk of colchicine side effects, including potentially fatal ones.
The use of the following drugs in combination with josamycin is not recommended:
— Ebastine
Increased risk of life-threatening arrhythmias in patients with congenital long QT syndrome.
— Dopamine receptor agonists (bromocriptine, cabergoline, lisuride, pergolide)
An increase in the concentration of dopamine receptor agonists in the blood plasma with a potential increase in their activity and the appearance of overdose symptoms.
— Triazolam
Several cases of increased side effects of triazolam (conduct disorder).
— Halofantrine
Increased risk of ventricular arrhythmias, including ventricular tachycardia of the “torsades de pointes” type. If possible, josamycin should be discontinued. If it is not possible to cancel concomitant use of drugs, monitoring of the QT interval and ECG is necessary.
— Disopyramide
Increased risk of side effects of disopyramide: severe hypoglycemia, increased duration of the QT interval and life-threatening arrhythmias, including ventricular tachycardia of the “pirouette” type. Monitoring of clinical and laboratory data, as well as regular ECG monitoring, is necessary.
— Tacrolimus
Increased plasma concentrations of tacrolimus and creatinine as a result of inhibition of tacrolimus metabolism in the liver.
— Terfenadine and astemizole
During combined use of josamycin and antihistamines containing terfenadine or astemizole, the risk of developing life-threatening arrhythmias may be increased.
The use of the following drugs together with josamycin requires caution:
— Carbamazepine
It is possible to increase the concentration of carbamazepine in the blood plasma and develop overdose symptoms due to inhibition of its hepatic metabolism. It is recommended to monitor the patient's condition and the concentration of carbamazepine in the blood plasma. A dose reduction of carbamazepine may be required.
— Cyclosporine
Co-administration of josamycin and cyclosporine may cause an increase in plasma levels of cyclosporine and creatinine and increase the risk of nephrotoxicity. Plasma concentrations of cyclosporine and renal function should be regularly monitored. The dose of cyclosporine should be adjusted during co-administration with josamycin, as well as after discontinuation of josamycin.
— Anticoagulants of indirect action
It is possible to enhance the effect of indirect anticoagulants and increase the risk of bleeding.
Frequent monitoring of the international normalized ratio (INR) is necessary. It may be necessary to reduce the dose of indirect anticoagulants during co-administration with josamycin, and also in some cases after discontinuation of josamycin.
— Sildenafil
It is possible to increase the concentration of sildenafil in the blood plasma, increasing the risk of arterial hypotension. If co-administration is necessary, it is recommended to take the lowest dose of sildenafil.
— Theophylline and aminophylline
Caution should be exercised when using josamycin together with theophylline or aminophylline, because there is a risk of increased plasma theophylline concentrations, especially in children.
— Digoxin
When administered together, josamycin and digoxin may increase the level of the latter in the blood plasma.
— Other antibacterial drugs
Since bacteriostatic antibiotics in vitro may reduce the antimicrobial effect of bactericidal antibiotics, their simultaneous use should be avoided. Josamycin should not be used simultaneously with lincosamides due to a possible mutual decrease in effectiveness.
Vilprafen 500mg/5ml 20g granules for suspension for oral administration
pharmachologic effect
Antibiotic, macrolide.
Composition and release form Vilprafen 500 mg/5 ml 20 g granules for the preparation of suspension for oral administration
Granules for preparing a suspension - 1 vial:
- Active ingredients: josamycin propionate - 6.307 g;
- Excipients: sodium citrate - 0.1125 g, methyl parahydroxybenzoate - 0.0795 g, propyl parahydroxybenzoate - 0.0105 g, simethicone - 0.075 g, hyprolose - 0.3 g, Avicel RC-591 [microcrystalline cellulose, sodium carmellose] - 0.6 g, strawberry flavor - 0.05 g, betacarotene - 0.015 g, sucrose starch powder 3% - 10.2005 g, mannitol - 2.25 g.
5 ml of the finished suspension contains 500 mg of josamycin.
15 g - Colorless glass bottles with a capacity of 100 ml (1) (complete with dosage syringe and syringe holder) - cardboard packs.
Description of the dosage form
Granules for the preparation of a suspension for oral administration are yellow, with the smell of strawberries; after dissolving in water, a yellow suspension with the smell of strawberries is formed.
Characteristic
Antibiotic of the macrolide group, produced by Streptomyces narbonensis var. Josamyceticus.
Directions for use and doses
When taken orally by adults and children over 14 years of age - 1-2 g/day in 2-3 divided doses. Children under 14 years of age - 30-50 mg/kg/day in 3 divided doses. The duration of treatment depends on the indications for use.
Pharmacodynamics
Antibiotic of the macrolide group. It has a bacteriostatic effect due to inhibition of protein synthesis by bacteria. When high concentrations are created at the site of inflammation, it has a bactericidal effect.
Highly active against intracellular microorganisms: Chlamydia trachomatis and Chlamydia pneumonuae, Mycoplasma pneumoniae, Mycoplasma hominis, Ureaplasma urealyticum, Legionella pneumophila; against gram-positive aerobic bacteria: Staphylococcus aureus, Streptococcus pyogenes and Streptococcus pneumoniae (pneumococcus), Corynebacterium diphtheriae; gram-negative aerobic bacteria: Neisseria meningitidis, Neisseria gonorrhoeae, Haemophilus influenzae, Bordetella pertussis; against some anaerobic bacteria: Peptococcus, Peptostreptococcus, Clostridium perfringens.
Josamycin is also active against Treponema pallidum.
Pharmacokinetics
After oral administration, josamycin is rapidly absorbed from the gastrointestinal tract. Cmax is achieved 1-2 hours after administration. 45 minutes after taking a dose of 1 g, the average concentration of josamycin in plasma is 2.41 mg/l.
Plasma protein binding does not exceed 15%.
An equilibrium state is achieved after 2-4 days of regular use.
Josamycin is well distributed in the body and accumulates in various tissues: in the lungs, lymphatic tissue of the palatine tonsils, organs of the urinary system, skin and soft tissues. Particularly high concentrations are determined in the lungs, tonsils, saliva, sweat and tear fluid. The concentration of josamycin in human polymorphonuclear leukocytes, monocytes and alveolar macrophages is approximately 20 times higher than in other cells of the body.
Josamycin is biotransformed in the liver to less active metabolites.
Excreted mainly in bile, excretion in urine is less than 20%.
Indications for use Vilprafen 500 mg/5 ml 20 g granules for the preparation of suspension for oral administration
Treatment of infectious and inflammatory diseases caused by microorganisms sensitive to josamycin: infections of the upper respiratory tract and ENT organs (including pharyngitis, tonsillitis, paratonsillitis, otitis media, sinusitis, laryngitis); diphtheria (in addition to treatment with diphtheria antitoxin); scarlet fever (with hypersensitivity to penicillin); lower respiratory tract infections (including acute bronchitis, bronchopneumonia, pneumonia, including atypical form, whooping cough, psittacosis); oral infections (including gingivitis and periodontal disease); infections of the skin and soft tissues (including pyoderma, boils, anthrax, erysipelas / with hypersensitivity to penicillin /, acne, lymphangitis, lymphadenitis); infections of the urinary tract and genital organs (including urethritis, prostatitis, gonorrhea; with increased sensitivity to penicillin - syphilis, lymphogranuloma venereum); chlamydial, mycoplasma (including ureaplasma) and mixed infections of the urinary tract and genital organs.
Contraindications
Severe liver dysfunction, hypersensitivity to erythromycin and other macrolide antibiotics.
Application of Vilprafen 500 mg/5 ml 20 g granules for the preparation of suspension for oral administration during pregnancy and lactation
Use during pregnancy and lactation is possible only in cases where the expected benefit to the mother outweighs the potential risk to the fetus or child.
When treating with macrolides and simultaneous use of hormonal contraceptives, non-hormonal contraceptives should be additionally used. Josamycin is not prescribed to premature babies. When used in newborns, it is necessary to monitor liver function.
special instructions
If pseudomembranous colitis develops, josamycin should be discontinued and appropriate therapy should be prescribed. Drugs that reduce intestinal motility are contraindicated.
In patients with renal failure, adjustment of the dosage regimen is required in accordance with CC values.
Josamycin is not prescribed to premature babies. When used in newborns, it is necessary to monitor liver function.
The possibility of cross-resistance to various macrolide antibiotics should be taken into account (for example, microorganisms that are resistant to treatment with antibiotics related in chemical structure may also be resistant to josamycin).
Overdose
Symptoms: increased side effects, especially from the gastrointestinal tract (there is no data on specific symptoms of poisoning).
Side effects Vilprafen 500mg/5ml 20g granules for suspension for oral administration
From the digestive system: rarely - lack of appetite, nausea, heartburn, vomiting, diarrhea, pseudomembranous colitis; in some cases - increased activity of liver transaminases, impaired bile outflow and jaundice.
Allergic reactions: rarely - urticaria.
Other: in some cases - dose-dependent transient hearing impairment.
Drug interactions
Bacteriostatic antibiotics may reduce the bactericidal effect of other antibiotics, such as penicillins and cephalosporins (simultaneous use of josamycin with penicillins and cephalosporins should be avoided).
With the simultaneous use of josamycin with lincomycin, the effectiveness of both drugs may be reduced.
Josamycin slows down the elimination of theophylline to a lesser extent than other macrolide antibiotics.
Josamycin slows the elimination of terfenadine or astemizole, which increases the risk of life-threatening arrhythmias.
There are isolated reports of increased vasoconstrictor effects with simultaneous use of macrolide antibiotics and ergot alkaloids. There was 1 case of ergotamine intolerance when taking josamycin.
With the simultaneous use of josamycin and cyclosporine, it is possible to increase the concentration of cyclosporine in the blood plasma up to nephrotoxic.
With the simultaneous use of josamycin and digoxin, an increase in the level of the latter in the blood plasma is possible.
In rare cases, during treatment with macrolides, the contraceptive effect of hormonal contraceptives may be insufficient.
Vilprafen granules d/prig. suspensions for oral administration 250mg/5ml fl 15g
Registration Certificate Holder
ASTELLAS PHARMA EUROPE (Netherlands)
Dosage form
Medicine - Wilprafen® (Wilprafen)
Description
Granules for preparation of suspension for oral administration
pink, with the smell of strawberries; after dissolving in water, a pink suspension with the smell of strawberries is formed.
1 fl.
josamycin propionate 3.1545 g
Excipients
: sodium citrate - 0.1125 g, methyl parahydroxybenzoate - 0.0795 g, propyl parahydroxybenzoate - 0.0105 g, simethicone - 0.075 g, hyprolose - 0.225 g, Avicel RC-591 [microcrystalline cellulose, sodium carmellose] - 1.2 g, strawberry flavor - 0.0375 g, canthaxanthin 10 % - 0.0075 g, starch powder sucrose - 7.848 g, mannitol - 2.25 g. 5 ml of the finished suspension contains 250 mg of josamycin.
15 g - Colorless glass bottles with a capacity of 100 ml (1) (complete with dosage syringe and syringe holder) - cardboard packs.
Indications
Treatment of infectious and inflammatory diseases caused by microorganisms sensitive to josamycin: infections of the upper respiratory tract and ENT organs (including pharyngitis, tonsillitis, paratonsillitis, otitis media, sinusitis, laryngitis); diphtheria (in addition to treatment with diphtheria antitoxin); scarlet fever (with hypersensitivity to penicillin); lower respiratory tract infections (including acute bronchitis, bronchopneumonia, pneumonia, including atypical form, whooping cough, psittacosis); oral infections (including gingivitis and periodontal disease); infections of the skin and soft tissues (including pyoderma, boils, anthrax, erysipelas / with hypersensitivity to penicillin /, acne, lymphangitis, lymphadenitis); infections of the urinary tract and genital organs (including urethritis, prostatitis, gonorrhea; with increased sensitivity to penicillin - syphilis, lymphogranuloma venereum); chlamydial, mycoplasma (including ureaplasma) and mixed infections of the urinary tract and genital organs.
Contraindications for use
Severe liver dysfunction, hypersensitivity to josamycin and other macrolide antibiotics.
pharmachologic effect
Antibiotic of the macrolide group. It has a bacteriostatic effect due to inhibition of protein synthesis by bacteria. When high concentrations are created at the site of inflammation, it has a bactericidal effect.
Highly active against intracellular microorganisms: Chlamydia trachomatis and Chlamydia pneumonuae, Mycoplasma pneumoniae, Mycoplasma hominis, Ureaplasma urealyticum, Legionella pneumophila; against gram-positive aerobic bacteria: Staphylococcus aureus, Streptococcus pyogenes and Streptococcus pneumoniae (pneumococcus), Corynebacterium diphtheriae; gram-negative aerobic bacteria: Neisseria meningitidis, Neisseria gonorrhoeae, Haemophilus influenzae, Bordetella pertussis; against some anaerobic bacteria: Peptococcus, Peptostreptococcus, Clostridium perfringens.
Josamycin is also active against Treponema pallidum.
Drug interactions
Bacteriostatic antibiotics may reduce the bactericidal effect of other antibiotics, such as penicillins and cephalosporins (simultaneous use of josamycin with penicillins and cephalosporins should be avoided).
With the simultaneous use of josamycin with lincomycin, the effectiveness of both drugs may be reduced.
Josamycin slows down the elimination of theophylline to a lesser extent than other macrolide antibiotics.
Josamycin slows the elimination of terfenadine or astemizole, which increases the risk of life-threatening arrhythmias.
There are isolated reports of increased vasoconstrictor effects with simultaneous use of macrolide antibiotics and ergot alkaloids. There was 1 case of ergotamine intolerance when taking josamycin.
With the simultaneous use of josamycin and cyclosporine, it is possible to increase the concentration of cyclosporine in the blood plasma up to nephrotoxic.
With the simultaneous use of josamycin and digoxin, an increase in the level of the latter in the blood plasma is possible.
In rare cases, during treatment with macrolides, the contraceptive effect of hormonal contraceptives may be insufficient.
Dosage regimen
When taken orally by adults and children over 14 years of age - 1-2 g/day in 2-3 divided doses. Children under 14 years of age - 30-50 mg/kg/day in 3 divided doses. The duration of treatment depends on the indications for use.
Side effect
From the digestive system:
rarely - lack of appetite, nausea, heartburn, vomiting, diarrhea, pseudomembranous colitis;
in some cases - increased activity of liver transaminases, impaired bile outflow and jaundice. Allergic reactions:
rarely - urticaria.
Other:
in some cases, dose-dependent transient hearing impairment.
special instructions
If pseudomembranous colitis develops, josamycin should be discontinued and appropriate therapy should be prescribed. Drugs that reduce intestinal motility are contraindicated.
In patients with renal failure, adjustment of the dosage regimen is required in accordance with CC values.
Josamycin is not prescribed to premature babies. When used in newborns, it is necessary to monitor liver function.
The possibility of cross-resistance to various macrolide antibiotics should be taken into account (for example, microorganisms that are resistant to treatment with antibiotics related in chemical structure may also be resistant to josamycin).
Use during pregnancy and breastfeeding
Restrictions during pregnancy - With caution. Restrictions when breastfeeding - With caution.
Use during pregnancy and lactation is possible only in cases where the expected benefit to the mother outweighs the potential risk to the fetus or child.
When treating with macrolides and simultaneous use of hormonal contraceptives, non-hormonal contraceptives should be additionally used.
Use for renal impairment
Restrictions for impaired renal function - With caution.
In patients with renal failure, adjustment of the dosage regimen is required in accordance with CC values.
Use for liver dysfunction
Restrictions for liver dysfunction - Contraindicated.
Contraindicated in severe liver dysfunction.
Use in children
Restrictions for children - With caution.
Josamycin is not prescribed to premature babies. When used in newborns, it is necessary to monitor liver function.