Home | About us | Delivery | Advertisers | Login | Registration
The pharmacy is closed on Sundays and holidays.
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2021 Pharmacy 84.
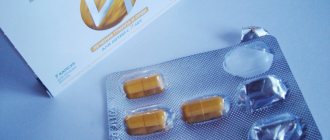
Bifren 250mg KPS No. 20
Trade name Bifren International nonproprietary name No Dosage form Capsules Composition One capsule contains the active substance - phenibut 250 mg excipients: microcrystalline cellulose, croscarmellose sodium, colloidal anhydrous silicon dioxide, magnesium stearate, talc; capsule composition: gelatin, titanium dioxide (E 171) Description White hard gelatin capsules. The contents of the capsules are white or almost white powder. The presence of compressed columns or lumps is allowed, which disintegrate when pressed . Pharmacotherapeutic group Psychoanaleptics. Psychostimulants and nootropics. Other psychostimulants and nootropics. Phenibut. Code ATXN06BX22 Pharmacological properties Pharmacokinetics The drug is well absorbed after oral administration and penetrates well into all tissues of the body, and penetrates well through the blood-brain barrier. Distribution in the liver and kidneys is close to uniform, but in the brain and blood it is less uniform. After 3 hours, a noticeable amount of administered phenibut is detected in the urine, at the same time the concentration of the drug in the brain tissue does not decrease; it is detected in the brain after another 6 hours. The next day, phenibut can only be detected in urine; it is found in urine 2 days after administration, but the detectable amount is only 5% of the administered dose. The greatest binding of phenibut occurs in the liver (80%) and is not specific. With repeated administration, cumulation is not observed. Pharmacodynamics Bifren is a derivative of γ-aminobutyric acid and phenylethylamine. Its dominant antihypoxic and antiamnestic effect is. Has tranquilizing properties, stimulates memory and learning, increases physical performance; eliminates psycho-emotional tension, anxiety, fear and improves sleep; prolongs and enhances the effect of sleeping pills, narcotics, neuroleptics and anticonvulsants. Does not affect cholinergic and adrenergic receptors. The drug lengthens the latent period and shortens the duration and severity of nystagmus, and has an antiepileptic effect. Noticeably reduces the manifestations of asthenia and vasovegetative symptoms, including headache, a feeling of heaviness in the head, sleep disturbance, irritability, emotional lability, and increases mental performance. Unlike tranquilizers, under the influence of the drug Bifren, psychological indicators (attention, memory, speed and accuracy of sensory-motor reactions) improve. In patients with asthenia and in emotionally labile patients, from the first days of therapy with the drug, their well-being improves, their interest and initiative, and motivation for vigorous activity without sedation or agitation increase. It has been established that phenibut improves the bioenergetics of the brain. Indications for use
- Asthenic and anxious-neurotic states: restlessness, fear, anxiety; in elderly patients – insomnia, night restlessness; prevention of stress before surgery
- Meniere's disease and dizziness associated with dysfunction of the vestibular analyzer of various origins
- Prevention of kinetosis (a specific disorder of the vestibular apparatus with characteristic symptoms: general malaise, nausea, vomiting associated with being on a moving vehicle, e.g. a boat or an airplane)
- Treatment of stuttering and tics in children
- An auxiliary agent in complex treatment for the relief of alcohol withdrawal syndrome
Directions for use and dosage : Take orally before eating with water. Adults are prescribed 250-500 mg 3 times a day. Highest single doses: for adults - 750 mg, for patients over 60 years old - 500 mg. The course of treatment is 4-6 weeks. Children over the age of 11 years - 250 mg 2-3 mg per day. Bifren can be combined with other psychotropic drugs, this increases its effectiveness. In this case, you can reduce the dose of Bifren and other concomitantly used drugs. To relieve alcohol withdrawal syndrome, Bifren in the first days of treatment is prescribed 250-500 mg 3 times a day and 750 mg at night, with a gradual reduction in the daily dose to the usual dose for adults. To eliminate dizziness due to dysfunction of the vestibular apparatus of infectious origin (otogenic labyrinthitis) and Meniere's disease during an exacerbation, Bifren is prescribed 750 mg 3 times a day for 5-7 days, with a decrease in the severity of vestibular disorders - 250-500 mg 3 times a day for 5-7 days and then - 250 mg 1 time per day for 5 days. For relatively mild diseases, Bifren is taken 250 mg 2 times a day for 5-7 days, and then 250 mg 1 time a day for 7-10 days. To eliminate dizziness due to dysfunction of the vestibular apparatus of vascular and traumatic origin, Bifren is prescribed 250 mg 3 times a day for 12 days. To prevent motion sickness during sea navigation, a dose of 250-500 mg is prescribed once, one hour before the expected start of motion sickness, when the first symptoms of seasickness appear. The effect of the drug Bifren increases with increasing dosage of the drug. In the presence of severe manifestations of seasickness (vomiting, nausea), the administration of the drug is ineffective even at a dose of 750-1000 mg. For complex treatment of women with osteochondrosis of the cervicothoracic spine and menopausal disorders, 250 mg 3 times a day is prescribed for the first 2 weeks, and 250 mg 2 times a day for the next 2 weeks. In case of moderate severity of vertebrogenic pain syndrome and menopausal disorders, it is recommended to use the drug Bifren in a dose of 500 mg (250 mg 2 times) daily for 4 weeks of complex treatment of osteochondrosis. If one or more doses have been missed, continue taking the previously prescribed doses; if necessary, or if the patient’s health worsens, the patient should consult a doctor. Side effects The frequency of side effects is determined according to the following criteria: very often (≥ 1/10), often (≥ 1/100 to < 1/10), infrequently (≥ 1/1000 to < 1/100), rare (≥ 1/10000 to < 1/1000), very rare (< 1/10000), frequency unknown (cannot be determined from available data). Rarely From the immune system: allergic reactions, including rash, itching, hives, redness of the skin. The frequency of manifestations is unknown (cannot be determined from the available data) From the nervous system: drowsiness (at the beginning of treatment), headache and dizziness (at doses above 2 g per day, when the dose is reduced, the severity of the side effect decreases). From the gastrointestinal tract: nausea (at the beginning of treatment), vomiting, diarrhea, pain in the epigastric region. From the liver and biliary tract: hepatotoxicity (with long-term use of high doses). Mental disorders: emotional lability, sleep disturbance (these adverse reactions can be observed in children when using the drug not in accordance with the instructions for use). Contraindications - hypersensitivity to the components of the drug - acute renal failure - children under 11 years of age Drug interactions Bifren can be used with other drugs, including tranquilizers and antipsychotics, since their effects can be mutually reinforcing. Special instructions Caution should be exercised in patients with pathologies of the digestive tract due to the irritant effect of Bifren. These patients are prescribed reduced doses. With long-term use, the cellular composition of the blood and indicators of functional liver tests are monitored. Children The drug can be used in children over 11 years of age. Use during pregnancy and lactation The use of the drug Bifren during pregnancy or breastfeeding is contraindicated, since there is insufficient data on the use of the drug during this period. The ability to influence the reaction rate when driving vehicles or other mechanisms Patients who experience drowsiness, dizziness or other disorders of the central nervous system during treatment with the drug should refrain from driving vehicles or operating other mechanisms. Overdose Bifren is a low-toxic compound; only in a daily dose of 7-14 g with long-term use it can be hepatotoxic (eosinophilia and fatty liver have been observed). Symptoms: drowsiness, nausea, vomiting, possible development of arterial hypotension, acute renal failure. Treatment: gastric lavage. Therapy is symptomatic. In case of complications (arterial hypotension, renal failure), auxiliary and symptomatic measures are used. Release form and packaging 10 capsules each in a blister pack made of polyvinyl chloride film and printed varnished aluminum foil. 2 blister packs together with instructions for medical use in the state and Russian languages are placed in a cardboard box. Storage conditions Store in original packaging at a temperature not exceeding 25 °C. Keep out of the reach of children! Shelf life: 2 years Do not use after expiration date Conditions for dispensing from pharmacies By prescription
Bifren 250 mg No. 20 caps.
APPROVED by the Order of the Chairman of the Pharmacy Committee of the Ministry of Health of the Republic of Kazakhstan Instructions for the medical use of the drug Bifren Trade name Bifren International nonproprietary name No Dosage form Capsules Composition One capsule contains the active substance - phenibut 250 mg excipients: microcrystalline cellulose, croscarmellose sodium, colloidal anhydrous silicon dioxide, magnesium stearate, talc; capsule composition: gelatin, titanium dioxide (E 171) Description White hard gelatin capsules. The contents of the capsules are white or almost white powder. The presence of compressed columns or lumps is allowed, which disintegrate when pressed. Pharmacotherapeutic group Psychoanaleptics. Psychostimulants and nootropics. Other psychostimulants and nootropics. Phenibut. ATC code N06B X22 Pharmacological properties Pharmacokinetics The drug is well absorbed after oral administration and penetrates well into all tissues of the body, penetrates well through the blood-brain barrier. Distribution in the liver and kidneys is close to uniform, but in the brain and blood it is less uniform. After 3 hours, a noticeable amount of administered phenibut is detected in the urine, at the same time the concentration of the drug in the brain tissue does not decrease; it is detected in the brain after another 6 hours. The next day, phenibut can only be detected in urine; it is found in urine 2 days after administration, but the detectable amount is only 5% of the administered dose. The greatest binding of phenibut occurs in the liver (80%) and is not specific. With repeated administration, cumulation is not observed. Pharmacodynamics Bifren is a derivative of γ-aminobutyric acid and phenylethylamine. Its dominant antihypoxic and antiamnestic effect is. Has tranquilizing properties, stimulates memory and learning, increases physical performance; eliminates psycho-emotional tension, anxiety, fear and improves sleep; prolongs and enhances the effect of sleeping pills, narcotics, neuroleptics and anticonvulsants. Does not affect cholinergic and adrenergic receptors. The drug lengthens the latent period and shortens the duration and severity of nystagmus, and has an antiepileptic effect. Noticeably reduces the manifestations of asthenia and vasovegetative symptoms, including headache, a feeling of heaviness in the head, sleep disturbance, irritability, emotional lability, and increases mental performance. Unlike tranquilizers, under the influence of the drug Bifren, psychological indicators (attention, memory, speed and accuracy of sensory-motor reactions) improve. In patients with asthenia and in emotionally labile patients, from the first days of therapy with the drug, their well-being improves, their interest and initiative, and motivation for vigorous activity without sedation or agitation increase. It has been established that phenibut improves the bioenergetics of the brain. Indications for use - Asthenic and anxious-neurotic conditions: restlessness, fear, anxiety; in elderly patients – insomnia, night restlessness; prevention of stress conditions before operations - Meniere's disease and dizziness associated with dysfunction of the vestibular analyzer of various origins - Prevention of kinetosis (a specific disorder of the vestibular apparatus with characteristic symptoms: general malaise, nausea, vomiting associated with being on a moving vehicle, e.g. a boat or an airplane) — Treatment of stuttering and tics in children — An auxiliary agent in complex treatment for the relief of alcohol withdrawal syndrome. Method of administration and dosage: Use orally before eating with water. Adults are prescribed 250-500 mg 3 times a day. Highest single doses: for adults - 750 mg, for patients over 60 years old - 500 mg. The course of treatment is 4-6 weeks. Children over the age of 11 years - 250 mg 2-3 mg per day. Bifren can be combined with other psychotropic drugs, this increases its effectiveness. In this case, you can reduce the dose of Bifren and other concomitantly used drugs. To relieve alcohol withdrawal syndrome, Bifren in the first days of treatment is prescribed 250-500 mg 3 times a day and 750 mg at night, with a gradual reduction in the daily dose to the usual dose for adults. To eliminate dizziness due to dysfunction of the vestibular apparatus of infectious origin (otogenic labyrinthitis) and Meniere's disease during an exacerbation, Bifren is prescribed 750 mg 3 times a day for 5-7 days, with a decrease in the severity of vestibular disorders - 250-500 mg 3 times a day for 5-7 days and then - 250 mg 1 time per day for 5 days. For relatively mild diseases, Bifren is taken 250 mg 2 times a day for 5-7 days, and then 250 mg 1 time a day for 7-10 days. To eliminate dizziness due to dysfunction of the vestibular apparatus of vascular and traumatic origin, Bifren is prescribed 250 mg 3 times a day for 12 days. To prevent motion sickness during sea navigation, a dose of 250-500 mg is prescribed once, one hour before the expected start of motion sickness, when the first symptoms of seasickness appear. The effect of Bifren increases with increasing dose of the drug. In the presence of severe manifestations of seasickness (vomiting, nausea), the administration of the drug is ineffective even at a dose of 750-1000 mg. For complex treatment of women with osteochondrosis of the cervicothoracic spine and menopausal disorders, 250 mg 3 times a day is prescribed for the first 2 weeks, and 250 mg 2 times a day for the next 2 weeks. In case of moderate severity of vertebrogenic pain syndrome and menopausal disorders, it is recommended to use the drug Bifren at a dose of 500 mg (250 mg 2 times) daily for 4 weeks of complex treatment of osteochondrosis. If one or more doses have been missed, continue taking the previously prescribed doses; if necessary, or if the patient’s health worsens, the patient should consult a doctor. Side effects The frequency of side effects is determined according to the following criteria: very often (≥ 1/10), often (≥ 1/100 to < 1/10), infrequently (≥ 1/1000 to < 1/100), rare (≥ 1/10,000 to < 1/1000), very rare (< 1/10,000), frequency unknown (cannot be determined from available data). Rarely From the immune system: allergic reactions, including rash, itching, hives, redness of the skin. The frequency of manifestations is unknown (cannot be determined from the available data) From the nervous system: drowsiness (at the beginning of treatment), headache and dizziness (at doses above 2 g per day, when the dose is reduced, the severity of the side effect decreases). From the gastrointestinal tract: nausea (at the beginning of treatment), vomiting, diarrhea, pain in the epigastric region. From the liver and biliary tract: hepatotoxicity (with long-term use of high doses). Mental disorders: emotional lability, sleep disturbance (these adverse reactions can be observed in children when using the drug not in accordance with the instructions for use). Contraindications - hypersensitivity to the components of the drug - acute renal failure - children under 11 years of age Drug interactions Bifren can be used with other drugs, including tranquilizers and antipsychotics, since their effects can be mutually reinforcing. Special instructions Caution should be exercised in patients with pathologies of the digestive tract due to the irritant effect of Bifren. These patients are prescribed reduced doses. With long-term use, the cellular composition of the blood and indicators of functional liver tests are monitored. Children The drug can be used in children over 11 years of age. Use during pregnancy and lactation The use of the drug Bifren during pregnancy or breastfeeding is contraindicated, since there is insufficient data regarding the use of the drug during this period. The ability to influence the reaction rate when driving vehicles or other mechanisms Patients who experience drowsiness, dizziness or other disorders of the central nervous system during treatment with the drug should refrain from driving vehicles or operating other mechanisms. Overdose Bifren is a low-toxic compound; only in a daily dose of 7-14 g with long-term use it can be hepatotoxic (eosinophilia and fatty liver have been observed). Symptoms: drowsiness, nausea, vomiting, possible development of arterial hypotension, acute renal failure. Treatment: gastric lavage. Therapy is symptomatic. In case of complications (arterial hypotension, renal failure), auxiliary and symptomatic measures are used. Release form and packaging 10 capsules each in a blister pack made of polyvinyl chloride film and printed varnished aluminum foil. 2 blister packs together with instructions for medical use in the state and Russian languages are placed in a cardboard box. Storage conditions Store in original packaging at a temperature not exceeding 25 °C. Keep out of the reach of children! Shelf life 2 years Do not use after the expiration date Conditions for dispensing from pharmacies By prescription Manufacturer/Registration Certificate Holder Pharma Start LLC, Ukraine, Kiev, blvd. I. Lepse, 8. Name, address and contact details of the organization on the territory of the Republic of Kazakhstan, accepting claims (suggestions) on the quality of medicines from consumers and responsible for post-registration monitoring of the safety of the medicine: Atsino Kaz LLP, Republic of Kazakhstan, 050010, Almaty, st. Begalina, 136A