Buy Ceraxon solution for internal use 100 mg/ml 30 ml in pharmacies
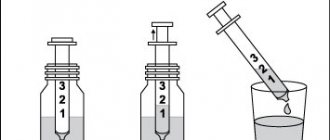
Oral solution in the form of a clear, colorless liquid with a characteristic strawberry odor. Indications : acute period of ischemic stroke (as part of complex therapy); — recovery period of ischemic and hemorrhagic strokes; — traumatic brain injury, acute (as part of complex therapy) and recovery period; — cognitive and behavioral disorders in degenerative and vascular diseases of the brain. Dosage regimen The drug is taken with meals or between meals. Before use, the drug can be diluted in a small amount of water (120 ml or 1/2 cup). Acute period of ischemic stroke and traumatic brain injury: the recommended dose is 1000 mg (10 ml or 1 sachet) every 12 hours. Duration of treatment is at least 6 weeks. Recovery period of ischemic and hemorrhagic strokes, recovery period of traumatic brain injury, cognitive and behavioral disorders in degenerative and vascular diseases of the brain: the recommended dose is 500-2000 mg/day (5-10 ml 1-2 times/day or 1 sachet ( 1000 mg) 1-2 times/day). The dose and duration of treatment depend on the severity of the symptoms of the disease. Elderly patients do not require dose adjustment of Ceraxon®. Rules for using a dosing syringe 1. Place the dosing syringe in the bottle (the syringe plunger is completely lowered). 2. Carefully pull the plunger of the dosing syringe until the solution level is equal to the corresponding mark on the syringe. 3. Before taking, the required amount of solution can be diluted in 1/2 glass of water (120 ml). After each use, it is recommended to rinse the dosing syringe with water. Rules for using the drug in sachets 1. Holding the sachet vertically, carefully tear off its edge along the o. 2.The contents of the sachet can be drunk immediately after opening or before use can be diluted in 1/2 glass of water (120 ml). Side effects Very rare (<1/10,000) (including individual cases): allergic reactions (rash, itching, anaphylactic shock), headache, dizziness, feeling of heat, tremor, nausea, vomiting, diarrhea, hallucinations, swelling, shortness of breath , insomnia, agitation, loss of appetite, numbness in paralyzed limbs, changes in the activity of liver enzymes. In some cases, Ceraxon® can stimulate the parasympathetic system and also cause a short-term change in blood pressure. If any of the side effects indicated in the instructions get worse, or any other side effects not listed in the instructions are noticed, you should inform your doctor. Contraindications for use : severe vagotonia (predominance of the tone of the parasympathetic part of the autonomic nervous system); - children and adolescents under 18 years of age (due to the lack of sufficient clinical data); - rare hereditary diseases associated with fructose intolerance; - hypersensitivity to the components of the drug. Use during pregnancy and lactation There is not enough clinical data on the use of citicoline during pregnancy. Although experimental studies on animals have not revealed any negative effects, during pregnancy the drug is prescribed only when the expected benefit of therapy for the mother outweighs the potential risk to the fetus. If it is necessary to use the drug during lactation, the issue of stopping breastfeeding should be decided, since there is no data on the excretion of citicoline in breast milk. Use in children Contraindicated in children and adolescents under 18 years of age. Use in elderly patients Elderly patients do not require dose adjustment of Ceraxon®. Special instructions In the solution for oral administration in the cold, a small amount of crystals may form due to temporary partial crystallization of the preservative. With further storage under recommended conditions, the crystals dissolve within several months. The presence of crystals does not affect the quality of the drug. Impact on the ability to drive vehicles and operate machinery During treatment, patients should be careful when performing potentially dangerous activities that require special attention and speed of psychomotor reactions (including driving a car and other vehicles, working with moving mechanisms, working as a dispatcher and operator). Overdose Due to the low toxicity of the drug, cases of overdose have not been described. Drug interactions Citicoline enhances the effects of levodopa. Ceraxon® should not be prescribed concomitantly with medications containing meclofenoxate. Conditions and periods of storage The drug should be stored out of the reach of children at a temperature not exceeding 30°C. Shelf life: 3 years. Conditions for dispensing from pharmacies The drug is dispensed with a prescription.
Citicoline: new therapeutic options
Currently, there is a significant increase in cerebrovascular pathology. Cerebrovascular diseases (CVD) are one of the main causes of mortality and permanent disability in patients. In clinical practice, drugs classified as “neuroprotectors” are widely used. The drug Ceraxon (citicoline), a natural endogenous nucleoside consisting of cytidine and choline linked by a diphosphate bridge, and participating in the synthesis of membrane phospholipids as an intermediate, has a targeted effect on the key links in the processes of death of nerve cells of vascular, traumatic, toxic and other etiologies from the group of neuroprotectors. link Citicoline not only restores damaged neuronal membranes, but also serves as a choline donor for the synthesis of acetylcholine [1]. Citicoline also inhibits the synthesis of phospholipase A2, reducing the accumulation of free fatty acids, restores the functioning of Na+/K+-ATPase, enhances the activity of antioxidant systems, prevents the processes of oxidative stress and apoptosis, has a positive effect on cholinergic transmission, modulates dopamine and glutamatergic neurotransmission. In addition, Ceraxon has a pronounced neuroreparative effect, stimulating the processes of neuro- and angiogenesis [2].
The formation of citicoline in neuronal membranes and phospholipid macrosomes is the rate-limiting step in the synthesis of phosphatidylcholine (lecithin); in this case, citicoline serves as a choline donor for the synthesis of acetylcholine and can limit the volume of the latter; oxidation produces betaine, a donor of methyl groups [3]. Since citicoline is a natural metabolite of biochemical processes in the body and combines neurotransmitter and neurometabolic effects in its spectrum of action. The most important of them is the activation of the biosynthesis of membrane phospholipids of brain neurons, primarily phosphatidylcholine.
Cognitive impairment is a key manifestation of dyscirculatory encephalopathy (DEP), which largely determines the severity of the patient’s condition. At the early stage of DEP, moderate neurodynamic disturbances predominate in the form of slowness, aspontaneity, decreased performance, exhaustion, and weakened concentration. Further progression of the cognitive defect in DEP is associated with the development of dementia, in which cognitive deficit leads to limitation of daily activities and at least partial loss of everyday independence. Treatment of patients with DEP is, in fact, limited to therapeutic effects on the manifestations of DEP stages I and II. The main directions of management of these patients are reversing the developed decompensation of the pathological process, preventing the progression of the disease, including stroke, reducing the severity of cognitive disorders and neurological deficits [4, 5].
Some studies devoted to the diagnosis and principles of treatment of cognitive disorders in vascular diseases of the brain provide a rationale for the prescription of citicoline for mild cognitive impairment (MCI) and dementia [6]. The authors conclude that the positive effects of citicoline affect all stages of the ischemic cascade, and the combination of neuroprotective and neurometabolic effects of the drug determines its ability to reduce beta-amyloid deposition in the brain. Citicoline is considered as a promising drug in the treatment of vascular forms of MCI, which also develop in patients taking ethanol for a long time.
Clinical studies in recent years have shown the neuroprotective effect of citicoline in acute cerebrovascular accident (ACVA), in the early recovery period, and CI therapy. Review articles on the drug citicoline provide data from foreign studies dating back to the 1980s. [3]. Analysis of the data obtained, based on the principles of evidence-based medicine, showed the effectiveness of citicoline in acute cerebral stroke. Citicoline is able to potentiate the effect of other drugs in the treatment of acute cerebrovascular pathology, including thrombolytics, antiplatelet agents and neurotrophics. In this regard, the prescription of citicoline is justified in complex therapy with other drugs that improve neurometabolism, such as Actovegin.
A study was conducted to evaluate the effectiveness of complex therapy with the drugs Ceraxon and Actovegin with the participation of 104 patients aged 55–80 years in the acute period of ischemic stroke [7]. Patients were divided into 4 groups: patients of group 1 (n = 25) received standard basic therapy; 2nd (n = 25) - for 10 days they additionally received citicoline (1000 mg/day, intravenously, drip into 200 ml of saline); 3rd (n = 26) - for 10 days they additionally received Actovegin (250 ml of a 20% solution intravenously, drips in saline); 4th (n = 28) - combination therapy with citicoline and Actovegin for 10 days. The dynamics of the condition were carried out on the 2nd, 7th, 10th and 30th days of stroke, the parameters of computed tomography of the brain (on the 5th day), neurological status, indicators of the US National Institute of Health stroke scale were assessed. Health, NIH), Rankin scale, Barthel index. The most pronounced effect of the therapy was obtained in the 4th group of patients receiving combination therapy with citicoline and Actovegin, compared with the control group: on the 5th day, a tendency was found towards a more significant decrease in the volume of ischemic brain damage in dynamics, by 10- On the 1st day there was a significant acceleration in the regression of neurological symptoms according to the NIH scale, and by the 30th day there was a significantly more significant functional recovery according to the Rankin scale and the Barthel index. It was found that in the 3rd group of patients receiving citicoline, the dynamics of indicators were similar to those in the 4th group (recovery according to the Rankin scale and Barthel index). Thus, the combined use of citicoline and Actovegin is most optimal, since it leads to a more complete regression of the neurological deficit and greater functional independence of the patient by the end of the acute period of stroke.
Review articles [8, 9] focus on improving cognitive functions under the influence of citicoline (optimal dose - 1 g/day) in the treatment of vascular dementia and dementia in neurodegenerative diseases (Alzheimer's disease, Parkinson's disease), in which the administration of citicoline slows down the development of cognitive deficiency [3]. It is specifically noted that when citicoline is prescribed 600 mg/day for 20 days as monotherapy in patients with Parkinson's disease, an improvement in motor function is also observed (decreased bradykinesia, rigidity, tremor). The use of citicoline in complex therapy of Parkinson's disease allows one to halve the required dose of L-DOPA and, accordingly, minimize the side effects of L-DOPA replacement therapy, and in the early stages of the disease, significantly delay the administration of L-DOPA [8]. It is also emphasized that, according to the results of numerous studies, the use of citicoline 750–3000 mg/day on the first day after traumatic brain injury (TBI), followed by a course of treatment for 14–20 days, significantly reduces the duration of the comatose period.
In vascular diseases of the brain, asthenic syndrome often occurs not in isolation, but in combination with other syndromes. A number of studies have shown the high effectiveness of the injectable form of citicoline, both as monotherapy and in complex therapy of acute and chronic cerebral ischemia and associated asthenic conditions [5]. In 2011, a study was conducted to evaluate the effectiveness and safety of citicoline in 60 patients aged 50–70 years who were diagnosed with stroke with MCI [10]. During the study, patients were divided into 2 groups: the main group (n = 30), who received citicoline along with basic therapy, and the control group (n = 30), who received only basic therapy. Citicoline was prescribed daily at a dose of 3 ml 2 times a day for 6 months. An analysis of the dynamics of indicators was carried out: neurological status and a battery of tests to assess frontal dysfunction, a clock drawing test, verbal associations, memorization and reproduction of 12 words, a study of visual memory), emotional status, assessment of quality of life, subjective assessment of the effectiveness of treatment. By the 3rd month of observation, patients taking citicoline showed an increase in strength in the limbs; after 6 months, a significant improvement in indicators was obtained on scales assessing neurological deficits (regression of CI, emotional-affective and behavioral disorders) [10].
A study was conducted to evaluate the effectiveness of citicoline in the recovery period of ischemic stroke in patients with CI [11]. 33 patients aged 46–82 years who had suffered an ischemic stroke were examined. According to neuropsychological examination, all patients were found to have mild or moderate CI in the form of memory, attention and thinking disorders. According to the severity of CI, patients were divided into two groups: 1st (n = 22) with moderate CI, citicoline was prescribed 1000 mg/day intravenously for 10 days; 2nd (n = 11) with milder CIs, citicoline was administered 500 mg/day intravenously for 10 days. Under the influence of treatment with citicoline at a dose of 500 or 1000 mg/day for 10 days, positive changes in the neurodynamic characteristics of cognitive functions (improved memory, thinking) were noted. The improvement in cognitive function was more significant in the citicoline 1000 mg/day group. No adverse drug reactions have been recorded when prescribing citicoline. Thus, the study showed the effectiveness and safety of the use of citicoline in the recovery period in patients with post-stroke CIs [11].
Review articles provide data on the optimal dosing regimen based on the results of clinical studies of the drug [12]: citicoline, administered at a dose of 1000 mg/day for 8 weeks, accelerates the regression of hemiplegia; intravenous administration of the drug at a dose of 750 mg/day for 10 days, starting from the first 48 hours after the onset of stroke symptoms, contributed to the restoration of motor and cognitive functions; with intravenous administration of 1 g for 14 days, a faster recovery of consciousness and a significant improvement in general condition and functional status were noted. It is noted that a relatively high functional status is achieved in 61.3% who took citicoline at a dose of 500 mg/day, 39.4% who took citicoline at a dose of 1000 mg/day, and 52.3% who took citicoline at a dose of 2000 mg/day. days The degree of improvement in the groups taking citicoline at a dose of 500 and 2000 mg/day was approximately the same.
A number of studies have shown that citicoline can correct CI already at the initial stages of its manifestations in patients with DEP. The high effectiveness of citicoline in the prevention and treatment of moderate cognitive disorders of vascular origin has been shown [4]. Its use promotes regression of CI, reduces concomitant emotional, affective and behavioral disorders. In addition, citicoline is able to potentiate the effect of other drugs in the treatment of acute cerebrovascular pathology, including thrombolytics, antiplatelet agents and neurotrophics [8]. Prescription regimens for citicoline have already been developed and are actively used: 1000 mg for 10 days intramuscularly or intravenously once a day, then an oral solution of citicoline 2 ml 3 times a day for 3 months [13], or daily at a dose of 3 ml 2 times a day for 6 months. The duration of neuroprotection can last up to 12 months [3].
Currently, the universality of the mechanisms of cell damage in various types of pathological processes, including exposure to ethanol, has been proven. The final link in inflammation and ischemia is a violation of redox processes, a violation of metabolism and energy supply to cells. Today, neurotrophicity, neuroprotection, neuroplasticity and neurogenesis are considered fundamental neurobiological processes involved in the implementation of endogenous protective activity, as well as in attempts to counteract pathophysiological damaging mechanisms and stimulate endogenous repair. Neuroprotection is defined as the continuous adaptation of a neuron to new functional conditions, as the key to reducing damage to brain tissue caused by ischemia, it acts at the level of a molecular cascade that causes dysfunction and death of neurons [14].
Clinical experience with the use of citicoline in alcoholism and drug addiction is not as extensive, although there is some evidence of its effectiveness in these conditions [2, 15]. Thus, in a randomized, double-blind study by A. Chinchilla et al. (1995) [16] studied the effects of citicoline in 20 patients with alcohol withdrawal syndrome. In the group of patients receiving citicoline, at the end of the study (after 2 months), there was a significant improvement in attention and concentration, as well as orientation in time and space. As the authors noted, this observation suggests that the drug may be useful in the treatment of chronic alcoholism.
A number of studies have shown the effectiveness of using citicoline in drug addicts, including those who used ethanol. Thus, PF Renshaw et al. (1999) [17] conducted a double-blind, placebo-controlled study to evaluate the effect of citicoline on improving the functional state of the central nervous system. The observation experience included 14 patients with cocaine addiction: the “citicoline” group (n = 6; 38.0 ± 6.1 years) and the “placebo” group (n = 8; 35.8 ± 6.5 years). Citicoline was prescribed in capsules 500 mg 2 times a day for a short course (2 weeks). Patients in this group also had a long history of ethanol use. The effect of citicoline on immediate drug cravings and subjective mood was assessed. During the study, according to the results of the “Cocaine Dependence” questionnaire, a significant (p = 0.002) decrease in craving for the drug was noted when comparing indicators “before and after treatment,” as well as a decrease in the level of anxiety (p = 0.013), improvement in mood (the desire to experience pleasant feelings) on a visual analogue scale (p = 0.07). Subjective scores after treatment in the “Need (belief) for cocaine” test in the placebo group were significantly higher (p = 0.057) than in the citicoline group. Significant (p = 0.046) changes were also noted after treatment in the citicoline group in the “Desire to use cocaine right now” and “Lack of control over cocaine use” tests. During the entire observation period, no adverse drug reactions (ADRs) were noted. The results of a pilot study suggest that citicoline may be an adjunct to primary treatment.
In another study, ES Brown et al. (2007) [14] conducted a 12-week, randomized, double-blind, placebo-controlled study of citicoline as an adjunctive treatment in patients with mania (hypomania) and cocaine dependence. The purpose of the study was to assess changes in declarative memory, mood, and craving (dependence) for cocaine. The study included 44 patients who were prescribed citicoline (n = 23; 42.1 ± 6.6 years) or placebo (n = 21; 40.7 ± 8.0 years) according to the regimen: starting with 1 table. (500 mg/day) followed by an increase to 2 tablets. (1000 mg/day) for two weeks, then 3 tablets. (1500 mg/day) for four weeks and 4 tablets. (2000 mg/day) for six weeks. Mood, cocaine craving, and ADRs were assessed every two weeks, and cognition every four weeks for 12 weeks. No adverse reactions were reported by patients. Based on the results of the Rey Auditory Verbal Learning Test, the authors concluded that citicoline could be considered a new approach in the treatment of drug addiction.
SJ Yoon et al. (2010) [18] conducted a longitudinal proton magnetic resonance spectroscopy study to evaluate changes in neurometabolite levels after 4 weeks of citicoline therapy in patients with methamphetamine (MA) addiction. The study involved 31 MA-dependent patients who received citicoline (n = 16; 38.6 ± 3.9 years) or placebo (n = 15; 38.3 ± 3.5 years) for four weeks. Based on the levels of N-acetylaspartate and choline in the prefrontal zone, it was found that changes in these markers were higher in the citicoline group than in the placebo group. No adverse reactions were noted. Based on the data obtained, the authors concluded that the results may indicate that citicoline therapy may have a direct neuroactive effect on reducing the dosage of drug use in MA dependence.
Recently, a double-blind, placebo-controlled study was conducted to evaluate the effects of citicoline on volunteers with cocaine addiction [19]. A total of 43 people (control group) and 29 participants with drug addiction (marijuana, alcohol) took part in the study, of which the citicoline group (n = 15; 38.4 ± 4.5 years) and placebo (n = 14; 39.0 ± 5.3 years). Citicoline was prescribed 500 mg/day 2 times a day. The course of treatment was 8 weeks, followed by a 4-week continuation of treatment in the group of cocaine addicts. During the study period, adverse reactions were noted (headache, feeling cold/sweating, muscle cramps, nausea/indigestion/diarrhea, trembling/shaking), which were observed in both groups, including in the control group. According to the results of patient self-reports over the entire observation period, there was an improvement in concentration, appetite, sleep quality, mainly in the citicoline group, as well as a decrease in the level of irritability and physical discomfort after therapy. However, there were no significant changes in the emotional domain (mood, anxiety, tension) either during the treatment period or during subsequent treatment, and there were also no significant differences between the citicoline and placebo groups. In each group (citicoline, placebo), in addition to marijuana use, patients had a history of alcoholism and tobacco smoking. These studies allowed the authors to suggest that citicoline can be used as an adjuvant (reducing the dose of use) in the treatment of alcohol and marijuana addiction. The results of this study should be interpreted with caution because it was not designed to assess the amount of alcohol or marijuana use among cocaine-dependent individuals.
Due to its stabilizing effect on the membrane, citicoline has an anti-edematous effect and prevents the development of cerebral edema, and by suppressing phospholipases A1, A2, C-D, it reduces the formation of free radicals and strengthens antioxidant defense systems. The above properties of the drug allow us to recommend citicoline for inclusion in the standard treatment regimen for patients with dizziness of central origin of vascular etiology [20]. It is well known that otoneurological symptoms are an integral part of the clinical picture of CVD and are observed both in the symptom complex of asymptomatic ischemic brain damage and in stroke of varying severity.
The relevance and medical and social significance of optimizing the treatment of dizziness of vascular origin are determined not only by its widespread prevalence, but also by the frequent chronicity of this condition, which requires significant effort [21]. Dizziness is one of the most common complaints; This is the second reason for visiting a doctor after a headache. It is estimated that dizziness can manifest itself in about 80 diseases: neurological, mental, cardiovascular, ophthalmological, otorhinolaryngological, etc. [22]. A common cause of dizziness in people of working age are osteochondrosis of the cervical spine and vertebrobasilar discirculation. A prerequisite for the development of vertebrobasilar discirculation can be deformations (pathological tortuosity, kinks) and anomalies (hypoplasia, anomalies in the origin, location and entry of arteries, etc.) of the vertebral arteries, which occur in 20–35% of cases in patients with vascular diseases of the brain [21] .
Dizziness in cerebrovascular diseases is caused by transient or persistent disruption of the blood supply to the central or peripheral parts of the vestibular system [22]. Dizziness (vertigo or dizziness) is an illusory sensation of movement of surrounding objects relative to the patient or movement of the patient relative to objects. The concept of dizziness also includes imbalance, “ringing/tinnitus”: all these symptoms significantly reduce the patient’s quality of life and ability to work. At the first stage of treatment of acute and chronic diseases of the vestibular analyzer, a combination of vasoactive, nootropic drugs, dehydrating drugs, anti-vertigo drugs and non-steroidal anti-inflammatory drugs is usually prescribed [20, 22]. Often such combination therapy is not effective enough, which requires increasing the doses of the drugs used and, thus, increasing the risk for patients.
Of particular interest is a study on the use of high doses of citicoline for dizziness, conducted between January and December 2010 in two major neurological clinics in Sofia, Bulgaria. This open-label study provided a comparative assessment of the short-term therapeutic effectiveness of citicoline in patients with central vertigo of vascular etiology. Citicoline was prescribed at a dosage of 2000 mg/day intravenously and 1000 mg/day intravenously against the background of insufficiently effective standard therapy [20]. The study included 40 patients (mean age 55.5 ± 4.2 years), who were divided into two groups: group 1 (n = 20) received citicoline at a dose of 2000 mg/day, group 2 (n = 20) - citicoline at a dose of 1000 mg/day. Treatment regimens: for group 1 - a dose of citicoline 2000 mg/day for 5 days, then orally 500 mg/day (2 ml + 2 ml + 1 ml) for up to 30 days, for group 2 - a dose of citicoline 1000 mg/day for 5 days, then orally 500 mg/day (2 ml + 2 ml + 1 ml) for up to 30 days. Course of treatment up to 5 days: citicoline was added to 100 ml of saline solution and administered intravenously for 30–60 minutes. Next, citicoline in both groups was prescribed orally for up to 30 days at a dose of 500 mg/day (2 ml + 2 ml + 1 ml, in each milliliter - 20 drops of a solution containing 100 mg of citicoline). The dynamics of the condition were assessed at certain “points” of the study: 1 - before the start of treatment with citicoline, 2 - 5 days after a course of parenteral treatment in the form of intravenous infusion at a daily dose of 1000 mg/day or 2000 mg/day of citicoline and 3 - on the 30th day after oral administration at a dose of 500 mg/day (2 ml + 2 ml + 1 ml). During the study, patients took only the appropriate anti-vertigo drug (betagistine hydrochloride) and a certain dose of citicoline. To assess the dynamics of the condition, the following were used: 1) a questionnaire containing a detailed otoneurological history; 2) neurological and otological status; 3) tone audiometry threshold; 4) study of the vestibular analyzer (spontaneous and evoked responses); 5) electroencephalography. Treatment with citicoline should be started with a dose of 2000 mg/day intravenously, since the therapeutic effect is achieved faster, and no significant differences in tolerability or NLR were observed compared with a dose of 1000 mg/day. The study found: on the 5th day of parenteral use of citicoline 2000 mg/day (group 1), the most pronounced positive effect was obtained for vertigo, dizziness and the subjective sensation of “tinnitus” (in approximately 1/2 of cases), while in group 2 (citicoline 1000 mg/day) - only in 1/3 of cases (vertigo: group 1 - 20% vs group 2 - 35%; dizziness 25% vs 40%; "tinnitus" - 25% vs 35%) . A significant effect was observed regarding the correction of instability in both groups - about half of all patients. On the 30th day of treatment, vertigo, dizziness, imbalance, and “tinnitus” persisted in only 1/3 of the patients, and the severity of symptoms decreased. The greatest effect on spontaneous and, in particular, latent nystagmus was noted on the 5th day of taking citicoline at a dose of 2000 mg/day. After the fifth day of treatment with citicoline at a dose of 2000 mg/day, an improvement in asymmetry (according to electronystagmography) was noted in more than 20% of patients compared to the group of patients receiving citicoline at a dose of 1000 mg/day. The improvement in subjective results was accompanied by objective changes in the examination parameters of the vestibular analyzer, the symmetry of the reaction, and the results of coordination and statokinetic tests. This fact allows us to recommend starting citicoline therapy with a dose of 2000 mg/day, since a high dose leads to a significant acceleration of the recovery of vestibular function. The results obtained allow us to postulate the following: initiation of parenteral therapy with citicoline at a dose of 2000 mg/day, lasting at least 5 days, then switching to citicoline 500 mg/day orally (2 ml + 2 ml + 1 ml) for a period of 1 to 3 months in as part of standard treatment for patients with dizziness of central origin of vascular etiology [20]. Thus, this study expands the indications for the use of citicoline as part of complex therapy in patients with dizziness.
Conclusion
A number of studies have shown the effectiveness of the use of the drug Ceraxon in the treatment of chronic cerebral ischemia accompanied by CI, in the treatment of vascular dementia and dementia in neurodegenerative diseases (Alzheimer's disease, Parkinson's disease). In addition, the use of Ceraxon in the complex therapy of Parkinson's disease allows one to halve the required dose of L-DOPA. The neuroprotective effect of citicoline has been shown in acute stroke, in the early recovery period, as well as in complex therapy with Actovegin. There is experience in using Ceraxon in drug addicted patients and people suffering from alcohol addiction. Recent studies have shown the therapeutic effectiveness of Ceraxon in patients with central dizziness of vascular etiology of parenteral therapy with Ceraxon at a dose of 2000 mg/day, lasting at least 5 days.
Literature
- Plataras C., Taskiris S., Angelogianni P. Effect of CDP-choline on brain acetylcholinesterase and Na+/K+-ATPase in adult rats // Clin Biochem. 2000; 33: 351–357.
- Secades JJ Citicoline: pharmacological and clinical review, 2010 update // Rev Neurol. 2011. Vol. 52(Suppl.2):S1-S62.
- Shavlovskaya O. A. Neuroprotective therapy of neurological deficit in cerebrovascular pathology // Practicing doctor today. 2012. No. 3. pp. 39–44.
- Shavlovskaya O. A. Neuroprotective therapy for chronic cerebral ischemia // Treating Doctor. 2013. No. 9. pp. 32–37.
- Shavlovskaya O. A. Therapy of asthenic conditions in patients suffering from chronic cerebral ischemia // Farmateka. 2013. No. 19 (272). pp. 56–61.
- Sorokina I. B., Gudkova A. A., Gekht A. B. Moderate cognitive impairment in cerebrovascular diseases: diagnosis and principles of therapy // Difficult Patient. 2010; 8 (3): 9–13.
- Shamalov N. A., Stakhovskaya L. V., Shetova I. M. et al. Study of the safety and effectiveness of combination therapy with citicoline and Actovegin for patients in the acute period of ischemic stroke // Journal of Neurology and Psychiatry named after. S. S. Korsakova. 2010. No. 9. Issue. 2. pp. 13–17.
- Boyko A. N., Kabanov A. A. Cytocoline: new possibilities of neuroprotection and pharmacotherapy for diseases of the nervous system // Farmateka. 2007, no. 15, p. 42–48.
- Rumyantseva S. A., Silina E. V., Svishcheva S. P., Shuchalin O. G., Koryukova I. V., Eliseev E. V. Complex neuroprotection in patients with vascular pathology of the brain // Breast Cancer. 2008; 16 (17): 1124–1129.
- Putilina M.V. Features of combined neuroprotective therapy for acute cerebrovascular accidents // Breast Cancer. 2009, vol. 17, no. 4, p. 261–267.
- Shakhparonova N.V., Kadykov A.S., Kashina E.M. Post-stroke cognitive impairment and their therapy with Ceraxon // Neurology, neuropsychiatry, psychosomatics. 2011, 3: 56–60.
- Shetova I.M., Shamalov N.A., Botsina A.Yu. Use of citicoline in the acute period of cerebral stroke // Journal of Neurology and Psychiatry named after. S. S. Korsakova. 2009. No. 12, issue. 2. pp. 51–54.
- Putilina M.V., Nakhlas M.S. The place of neuroprotective therapy in the early rehabilitation period of ischemic stroke // Treatment of diseases of the nervous system. 2011; 3 (8): 26–31.
- Brown ES, Gorman AR, Hynan LS A randomized, placebo-controlled trial of citicoline add-on therapy in outpatients with bipolar disorder and cocaine dependence // J Clin Psychopharmacol. 2007. Vol. 27. R. 498–502.
- Shavlovskaya O. A. Possibilities of treating post-alcohol changes in the nervous system // Treating Doctor. 2014. No. 5. pp. 24–29.
- Chinchilla A., Lopez-Ibor JJ, Vega M., Camarero M. CDP-colina en la evolucion de las funciones mentales en el sindrome de abstinence alcoholica // Psiquiatria Biologica. 1995; 2: 171–175.
- Renshaw PF, Daniels S, Lundahl LH et al. Short-term treatment with citicoline (CDP-choline) attenuates some measures of craving in cocaine-dependent subjects: a preliminary report // Psychopharmacology. 1999. Vol. 142. P. 132–138.
- Yoon SJ, Lyoo IK, Kim HJ Neurochemical alterations in methamphetamine-dependent patients treated with cytidine-50-diphosphate choline: a longitudinal proton resonance magnetic spectroscopy study // Neuropsychopharmacology. 2010. Vol. 35. P. 1165–1173.
- Licata SC, Penetar DM, Ravichandran C. et al. Effects of daily treatment with citicoline: A double-blind, placebo-controlled study in cocaine-dependent volunteers // J Addict Med. March 2011; 5(1):57–64. doi:10.1097/ADM.0 b013 e3181 d80 c93; J Addict Med. Author manuscript; available in PMC 2012 March 1.
- Petrova D., Maslarov D., Angelov I., Zekin D. Analysis of therapeutic efficacy of citicoline in patients with vertigo of central origin and vascular aetiology // Am. J. Neuroprotec. Neuroregen. 2012. Vol. 4. No. 1: 1–8.
- Morozova S.V., Zaitseva O.V., Naletova N.A. Dizziness as a medical and social problem // RMJ. 2002. No. 16. pp. 725–729.
- Zamergrad M.V. “Vascular” dizziness // RMJ. 2007. No. 9. pp. 769–772.
O. A. Shavlovskaya, Doctor of Medical Sciences
GBOU VPO First Moscow State Medical University named after. I. M. Sechenova Ministry of Health of the Russian Federation, Moscow
Contact Information