Pharmacodynamics and pharmacokinetics
Pharmacodynamics
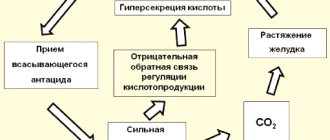
The drug belongs to the group of drugs derived from nitrofuran and has antimicrobial and antiprotozoal effects. Effective against staphylo / streptococci , gram-negative bacilli . of Proteus strains are resistant to the drug . The mechanism of action is due to inhibition of the process of nucleic acid .
The drug suppresses the activity of the Krebs cycle and the respiratory chain in microorganisms, inhibits the most important biochemical processes, which destroys the cytoplasmic or shell of microorganisms. Nitrofurans increase the ability of leukocytes to phagocytose pathological microorganisms.
Pharmacokinetics
Due to the lack of significant entry of the active substance of the drug into the systemic circulation, pharmacokinetics are not presented.
Furasol 0.1g 1g 15 pcs. powder for preparing solution
pharmachologic effect
Antimicrobial drug, nitrofuran derivative.
Effective against gram-positive cocci (Staphylococcus spp., Streptococcus spp.), gram-negative bacilli (Escherichia coli, Salmonella spp., Shigella spp., Klebsiella spp.). Acinetobacter spp., most strains of Proteus spp., Serratia spp. are resistant. The mechanism of action is associated with inhibition of nucleic acid synthesis. Depending on the concentration, it has a bactericidal or bacteriostatic effect. Against most bacteria, the bacteriostatic concentration is 10-20 μg/ml. The bactericidal concentration is approximately 2 times higher.
Under the influence of nitrofurans in microorganisms, the activity of the respiratory chain and the tricarboxylic acid cycle (Krebs cycle) is suppressed, as well as other biochemical processes are inhibited, which leads to the destruction of their shell or cytoplasmic membrane. Nitrofurans increase complement titer and the ability of leukocytes to phagocytose microorganisms.
Composition and release form Furasol 0.1g 1g 15 pcs. powder for preparing solution
Powder for preparing a solution for local and external use - 1 pack:
- Active substance: potassium furazidin - 100 mg;
- Excipients: sodium chloride - 900 mg.
Maminate sachets (15 pcs.), 1 g.
Description of the dosage form
Powder for preparing a solution for local and external use, orange-brown in color, large.
Pharmacokinetics
Data on the pharmacokinetics of the drug Furasol are not provided.
Indications for use Furasol 0.1g 1g 15 pcs. powder for preparing solution
With complex treatment:
- Infectious and inflammatory diseases of the oral cavity, oropharynx;
- small wounds with a risk of infection (including abrasions, scratches, small cuts and scratches, cracks, mild burns).
Contraindications
- Allergic dermatitis;
- pregnancy;
- lactation period (breastfeeding);
- children under 4 years of age;
- hypersensitivity to furazidine or nitrofuran drugs.
Application Furasol 0.1g 1g 15 pcs. powder for preparing a solution during pregnancy and breastfeeding
The use of the drug during pregnancy and lactation is contraindicated.
Do not use on children under 4 years of age.
special instructions
If adverse reactions develop, the drug should be discontinued and symptomatic therapy prescribed.
The solution is prepared immediately before use. The prepared solution should not be stored.
Impact on the ability to drive vehicles and machinery.
The use of the drug does not affect the ability to drive vehicles and machinery.
Overdose
The drug is low toxic. Cases of overdose have not been described.
Side effects Furasol 0.1g 1g 15 pcs. powder for preparing solution
Classification of adverse reactions by frequency of development: very often (≥10%), often (≥1%, but
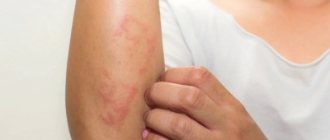
Allergic reactions: frequency unknown - urticaria, itching, rash, allergic dermatitis.
If unusual reactions occur, the patient should consult a doctor about the advisability of further use of the drug.
Drug interactions
In combination with antibiotics it exhibits synergism.
If you drink alcohol during treatment with the drug, the likelihood of developing allergic skin reactions increases.
Analogues of Furasol
Level 4 ATC code matches: Oflocain
Metrogyl
Chlorophyllipt
Oflomelid
Rozamet
Furagin , Furamag , Furazidine sodium , Sodium chloride , Magnesium carbonate .
Furasol powder for the preparation of local and external solution 100 mg N 15
Release form, composition and packaging
Powder for preparing a solution for local and external use, orange-brown in color, large.
| 1 pack (1 g) | |
| furazidine potassium | 100 mg |
Excipients: sodium chloride - 900 mg.
1 g - laminate bags (15) - cardboard packs.
Clinical and pharmacological group:
Antibacterial and antiprotozoal drug, nitrofuran derivative, for external and local use
Pharmacotherapeutic group:
Antimicrobial agent, nitrofuran derivative
pharmachologic effect
Antimicrobial drug, nitrofuran derivative. Effective against gram-positive cocci (Staphylococcus spp., Streptococcus spp.), gram-negative rods (Escherichia coli, Salmonella spp., Shigella spp., Klebsiella spp.). Resistant are Acinetobacter spp., most strains of Proteus spp., Serratia spp.
The mechanism of action is associated with inhibition of nucleic acid synthesis. Depending on the concentration, it has a bactericidal or bacteriostatic effect. Against most bacteria, the bacteriostatic concentration ranges from 10-20 μg/ml. The bactericidal concentration is approximately 2 times higher.
Under the influence of nitrofurans in microorganisms, the activity of the respiratory chain and the tricarboxylic acid cycle (Krebs cycle) is suppressed, as well as other biochemical processes are inhibited, which leads to the destruction of their shell or cytoplasmic membrane. Nitrofurans increase complement titer and the ability of leukocytes to phagocytose microorganisms.
Pharmacokinetics
Data on the pharmacokinetics of the drug Furasol® are not provided.
Indications
With complex treatment:
— infectious and inflammatory diseases of the oral cavity, oropharynx;
- small wounds with a risk of infection (including abrasions, scratches, small cuts and scratches, cracks, mild burns).
Dosage regimen
The drug is intended for local and external use.
The solution is prepared immediately before use. The contents of the sachet are dissolved in 200 ml of hot water.
Locally: a warm solution is used to rinse the mouth and oropharynx 2-3 times a day.
Externally: a warm solution is used to wash wounds 1-2 times a day.
Duration of treatment is 3-5 days.
Side effect
Classification of adverse reactions by frequency of development: very often (≥10%), often (≥1%, but <10%), infrequently (≥0.1%, but <1%), rarely (≥0.01%, but <0.1%) , very rare (<0.01%), frequency unknown (cannot be determined from available data).
Allergic reactions: frequency unknown - urticaria, itching, rash, allergic dermatitis.
If unusual reactions occur, the patient should consult a doctor about the advisability of further use of the drug.
Contraindications for use
- allergic dermatitis;
- pregnancy;
- lactation period (breastfeeding);
- children under 4 years of age;
- hypersensitivity to furazidine or nitrofuran drugs.
Use during pregnancy and breastfeeding
The use of the drug during pregnancy and lactation is contraindicated.
Use in children
Do not use on children under 4 years of age.
special instructions
If adverse reactions develop, the drug should be discontinued and symptomatic therapy prescribed.
The solution is prepared immediately before use. The prepared solution should not be stored.
Impact on the ability to drive vehicles and operate machinery
The use of the drug does not affect the ability to drive vehicles and machinery.
Overdose
The drug is low toxic. Cases of overdose have not been described.
Drug interactions
In combination with antibiotics it exhibits synergism.
If you drink alcohol during treatment with the drug, the likelihood of developing allergic skin reactions increases.
Conditions for dispensing from pharmacies
The drug is approved for use as a means of OTC.
Furasol price, where to buy
The price of Furasol powder in packages of 100 mg/1 g, No. 15 varies from 385 to 520 rubles per package. You can buy Furasol in most pharmacies in Moscow without difficulty.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
ZdravCity
- Furasol powder for local injection solution.
and outside approx. 100 mg 15 pcs. Olainfarm JSC 487 rub. order
Pharmacy Dialogue
- Furasol (portable d/pig solution for place/external ext. 100 mg package No. 15) Olainfarm OA
RUB 493 order
show more
Pharmacy24
- Furasol 0.1 g No. 15 powder AT "Olainfarm", Latvia
190 UAH. order
PaniPharmacy
- Furasol Furasol por. d/p solution 0.1g/package No. 15 Latvia, Olainfarm JSC
197 UAH order
show more
Furasol powder for solution for external use 100 mg in bags No. 5
Product description
The powder for preparing a solution for external use is large, orange-brown in color.
Compound
1 pack (1 g) soluble furagin 100 mg Excipients: sodium chloride - 900 mg. 1 g - laminate bags - cardboard packs.
Pharmacotherapeutic group
Antibacterial and antiprotozoal drug, nitrofuran derivative, for external and local use
pharmachologic effect
Antibacterial and antiprotozoal drug, a nitrofuran derivative. Active against gram-positive cocci (Staphylococcus spp., Streptococcus spp.), gram-negative rods (Escherichia coli, Salmonella spp., Shigella spp., Klebsiella spp.). Pseudomonas aeruginosa, Enterococcus spp., Acinetobacter spp., most strains of Proteus spp., Serratia spp. are resistant to the drug. The mechanism of action is associated with inhibition of nucleic acid synthesis. Depending on the concentration, it has a bactericidal or bacteriostatic effect. For most bacteria, the bacteriostatic concentration ranges from 10-20 μg/ml. The bactericidal concentration is approximately 2 times higher. Under the influence of nitrofurans in microorganisms, the activity of the respiratory chain and the tricarboxylic acid cycle (Krebs cycle) is suppressed, as well as other biochemical processes are inhibited, which leads to the destruction of their shell or cytoplasmic membrane. Nitrofurans increase complement titer and the ability of leukocytes to phagocytose microorganisms. Furagin soluble is effective in the treatment of external purulent infections. When applied to the skin and mucous membranes, the drug does not cause irritation or pain.
Indications for use
As part of complex therapy: infectious and inflammatory diseases of the oral cavity; infectious and inflammatory diseases of the oropharynx; infected wounds.
Dosage regimen
For external use. The solution is prepared immediately before use. The contents of the sachet are dissolved in 200 ml of hot water, and the warm solution is used to rinse the mouth and oropharynx 2-3 times a day. The drug solution is also used for washing wounds; after surgical treatment, apply directly to the wound surface, after which a sterile gauze bandage is applied, or applied to a dressing material, and then to the wound. Apply to the wound surface 2-3 times/day.
Side effects
In some cases, allergic reactions may develop.
Contraindications for use
allergic dermatitis; pregnancy; lactation period (breastfeeding); children under 4 years of age; hypersensitivity to soluble furagin or other nitrofuran drugs.
Use during pregnancy and breastfeeding
The use of the drug is contraindicated during pregnancy and lactation (breastfeeding).
Use in children
Not prescribed for children under 4 years of age
special instructions
If allergic reactions occur, use of Furasol should be stopped immediately. Antihistamines, calcium chloride and B vitamins are prescribed. The drug solution is prepared immediately before use. The prepared solution should not be stored. The solution turns the skin yellow.
Impact on the ability to drive vehicles and operate machinery
The drug does not affect the ability to drive a vehicle or maintain machinery.
Overdose
The drug is low toxic. Cases of overdose have not been described.
Drug interactions
In combination with antibiotics it exhibits synergism. If you drink alcohol during treatment with the drug, the likelihood of developing allergic skin reactions increases.
Conditions for dispensing from pharmacies
The drug is approved for use as a means of OTC.
Storage conditions of the drug
The drug should be stored in a dry place, protected from light, at a temperature of 15° to 25°C.
Shelf life of the drug
Shelf life: 4 years.
Buy Furasol por.d/prig.solution d/nar.prim.in pack 100mg in pack No. 5 in the pharmacy
Price for Furasol por.d/prig.solution d/nar.prim.in pack.100mg in pack No.5
Instructions for use for Furasol powder.