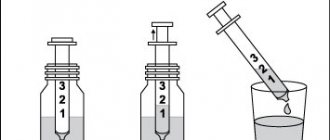
Principle of action of Gliatilin
The action of the drug is aimed at neural connections in the human brain. After being absorbed into the blood, the active substance, choline alfoscerate, reaches the brain, where it enters into chemical reactions. As a result of the formation of bonds, glycerol phosphate and choline are released. During the biotransformation process, phosphatidylcholine and acetylcholine are synthesized. The first substance increases the elasticity of cell membranes and their permeability. And the second substance is one of the most important nerve mediators.
Taking Gliatilin activates blood circulation processes in the brain, which ensures rapid restoration of lost functions. Most of the substance is eliminated by the respiratory system, along with carbon dioxide. Residues - with waste products.
Gliatilin solution for intravenous use 600 mg/7 ml bottle 7 ml No. 10
Compound
Active ingredients: choline alfoscerate 600 mg.
Excipients: methyl parahydroxybenzoate 8 mg, propyl parahydroxybenzoate 2.4 mg, sodium saccharinate 3 mg, orange flavor 29 mg, purified water up to 7 ml.
Pharmacokinetics
Absorption when taken orally is approximately 95%. Penetrates the blood-brain barrier (concentration in the brain is 45% of that in plasma). Both choline and glycerophosphate ion are included in general metabolism, so it is not possible to track their actual elimination from the body. However, they are known to be metabolized to carbon dioxide, water, phosphates and nitrogen-containing products.
Indications for use
As part of complex therapy:
- Psychoorganic syndrome against the background of degenerative diseases and involutional processes of the brain or cerebrovascular insufficiency, including primary and secondary senile cognitive impairment.
- Multi-infarct dementia.
Contraindications
- Hypersensitivity to the components of the drug;
- hemorrhagic stroke (acute stage);
- pregnancy;
- breastfeeding, childhood.
Directions for use and doses
Orally before meals, with water, 1 bottle of 600 mg (7 ml) 2 times a day. The duration of therapy is 3-6 months.
Storage conditions
At a temperature not higher than 25 °C. Keep out of the reach of children.
special instructions
3 years. Do not use after expiration date.
Description
Nootropic drug.
Pharmacodynamics
Choline alfoscerate (L-α-glycerylphosphorylcholine) is a prodrug from which choline, a precursor of acetylcholine, is released by hydrolysis.
The latter is an agonist of all subtypes of cholinergic receptors. Choline alfoscerate contains 40.5% choline by weight, released from. connections in the brain; Choline is involved in the biosynthesis of acetylcholine, is a donor of methyl groups, and is involved in other plastic reactions in the body. Alphoscerate ion is metabolized to glycerophosphate ion.
Acetylcholine is directly involved in the transmission of nerve impulses in both the central and peripheral nervous systems. Glycerophosphate is involved in various metabolic pathways, including participation in the synthesis of phosphatidylcholine (one of the phospholipids of cell membranes). Thus, the drug acts on the cholinergic transmission of nerve impulses and the plasticity of the neuron membrane.
The drug is believed to reduce cognitive impairment in degenerative and vascular lesions of the brain (including cerebrovascular insufficiency and some forms of dementia).
Side effects
From the digestive system: gastritis, gastric ulcer, constipation, diarrhea, dry oral mucosa, pharyngitis.
From the nervous system: headache, drowsiness, insomnia, aggressiveness, nervousness, cerebral ischemia, convulsions, hyperkinesia, dizziness.
From the skin: rash.
Other: increased urination, allergic reactions.
Use during pregnancy and breastfeeding
The use of the drug during pregnancy and breastfeeding is contraindicated.
Interaction
No drug interactions have been established.
Overdose
There is no information about overdose.
In case of overdose, the following are indicated: gastric lavage, taking adsorbent drugs (for example, activated carbon), symptomatic therapy. The effectiveness of dialysis has not been established.
Impact on the ability to drive vehicles and operate machinery
Caution should be exercised when driving vehicles or engaging in other activities that require increased concentration and/or speed of psychomotor reactions.
Indications for the use of Gliatilin
Gliatilin is prescribed for diagnosed disorders of the blood supply to the brain, which can be manifested by confusion, memory loss and other typical symptoms. Diseases and conditions that are direct indications for taking the drug:
- traumatic brain injuries leading to impaired consciousness, post-traumatic coma, dizziness;
- ischemic stroke, leading to necrosis of parts of the brain;
- dementia;
- age-related speech disorders;
- bouts of disorientation.
Patients taking gliatilin for long courses showed improvement in their condition, restoration of thought processes, and return of memory.
Instructions for use of Gliatilin
The instructions recommend long-term use of the drug, since restoration of brain function takes time. The minimum course is 3 months. It can be extended up to six months. As a rule, patients are prescribed the drug in the form of capsules for oral administration. But due to the fact that the capsules themselves are quite large, not every patient can absorb them, for example:
- child;
- a patient with a swallowing reflex disorder;
- patients who have suffered an ischemic stroke with a high degree of severity of damage.
In addition, unconscious and comatose patients are also unable to swallow the drug. In such cases, an alternative is prescribed - a solution for drip administration. 1 ampoule is diluted with 50 ml of saline solution. The rate of administration is no more than 80 drops per minute.
When prescribing capsules containing 400 mg of the active substance, the course consists of two to three doses per day. The medicine should be taken after meals. Each capsule is covered with a dense layer of soluble shell, which protects the active components from the aggressive environment of gastric juice and duodenum. Once in the large intestine, the coating disintegrates and releases the drug, from where it is easily absorbed into the bloodstream and reaches the brain. Therefore, the idea of cutting the capsule and squeezing the contents into a puree-like food to make it easier to take is impractical. The acidic environment of the stomach will destroy all the benefits of the substance.
Delecite solution for internal use 600 mg 7 ml No. 10
Additional information: Drug type medicinal product
Release form: oral solution
Prescription drug yes
Minimum age of use : 18 years
Purpose to improve memory
Indications for use As part of complex therapy: - Psychoorganic syndrome against the background of degenerative diseases and involutional processes of the brain or cerebrovascular insufficiency, including primary and secondary senile cognitive impairment. — Multi-infarct dementia.
Contraindications Hypersensitivity to the components of the drug, hemorrhagic stroke (acute stage), pregnancy; breastfeeding, childhood.
Use during pregnancy and breastfeeding Contraindicated.
Impact on the ability to drive vehicles and machinery Care should be taken when driving vehicles or engaging in other activities that require increased concentration and/or speed of psychomotor reactions.
Active ingredient: Choline alfoscerate
Method of administration and dosage: Orally before meals with water, 1 bottle of 600 mg (7 ml) 2 times a day. The duration of therapy is 3-6 months.
Side effects From the digestive system: gastritis, gastric ulcer, constipation, diarrhea, dry oral mucosa, pharyngitis. From the nervous system: headache, drowsiness, insomnia, aggressiveness, nervousness, cerebral ischemia, convulsions, hyperkinesia, dizziness. From the skin: rash. Other: increased urination, allergic reactions.
Storage conditions : At a temperature not exceeding 25 °C. Keep out of the reach of children!
Overdose There is no information about overdose. In case of overdose, the following are indicated: gastric lavage, taking adsorbent drugs (for example, activated carbon), symptomatic therapy. The effectiveness of dialysis has not been established.
Interaction No drug interactions have been established.
Pharmacological action Cholinomimetic. It is a precursor of acetylcholine. It affects primarily cholinergic receptors in the central nervous system. Glycerophosphate, which is formed by the breakdown of choline alphoscerate, is a precursor of phospholipids (phosphatidylcholine) of the neuron membrane. Facilitates the transmission of nerve impulses in cholinergic neurons, improves the plasticity of neuronal membranes and receptor function.
Pharmacological group Nootropic drug
Composition One 7 ml bottle contains: Active substance: choline alfoscerate - 600 mg; Excipients: methyl parahydroxybenzoate - 8.0 mg; propyl parahydroxybenzoate - 2.4 mg; sodium saccharinate - 3.0 mg; orange flavor - 29.0 mg; purified water - up to 7 ml.
Conditions for dispensing from a pharmacy By prescription.
Source : Vidal Medicinal Directory
Registration number LP-001540
State registration date 10.10.2016
Price for the drug Gliatilin
In the absence of Gliatilin in the pharmacy, the drug can be replaced with no less effective analogues with the same active ingredient:
- Choline alfoscerate. Sold in the form of a solution for intramuscular or drip administration. Each ampoule contains 250 mg/ml of active substance, ampoules are 4 ml each. There are 10 pieces in a package. The cost of this analogue is slightly higher than 550 rubles per package.
- Nooholin Rimpharm. Ampoules do not differ in volume and content of active substance. There are usually 3 ampoules in a package, the cost of the drug in the described dosage is from 350 rubles per package.
- Cereton. Available in capsules, each dose unit contains 400 mg of the active ingredient. A package of 56 capsules costs an average of 1,400 rubles. The solution for intravenous and intramuscular administration is packaged in 4 ml ampoules, each of which contains 0.25 ml of the substance. A pack of 5 ampoules can be purchased for 580 rubles.
- Holitylin 400 mg. Sold in capsules, 28 or 14 pieces per package. The dosage and number of doses does not differ from the prescribed regimen for taking Gliatilin. The cost of a package containing 14 doses varies from 405 rubles.
- Cerepro. It can be found both in ampoules and capsules. The amount of active substance in 1 dose does not differ from what is contained in analogues. A package of 28 capsules is sold at a price of 1,000 rubles.
Gliatilin 600mg/7ml 7ml 10 pcs. oral solution
pharmachologic effect
Nootropic drug.
Composition and release form Gliatilin 600 mg/7 ml 7 ml 10 pcs. oral solution
Solution - 1 fl. (7 ml):
- Active substance: choline alfoscerate - 600 mg;
- Excipients: methyl parahydroxybenzoate - 8.0 mg; propyl parahydroxybenzoate - 2.4 mg; sodium saccharinate - 3.0 mg; orange flavor - 29.0 mg; purified water - up to 7 ml.
7 ml of solution in brown glass bottles (type III, Evr.F.) sealed with polyethylene caps with first opening control. 10 bottles each along with instructions for use in a cardboard pack.
Description of the dosage form
Transparent colorless solution.
Directions for use and doses
Orally before meals, with water, 1 bottle of 600 mg (7 ml) 2 times a day. The duration of therapy is 3-6 months.
Pharmacodynamics
Improves the transmission of nerve impulses in cholinergic neurons; has a positive effect on the plasticity of neuronal membranes and receptor function. Improves cerebral blood flow, enhances metabolic processes in the brain, activates the structures of the reticular formation of the brain and restores consciousness in case of traumatic brain injury.
It has a preventive and corrective effect on such pathogenetic factors of involutional psychoorganic syndrome as changes in the phospholipid composition of neuronal membranes and a decrease in cholinergic activity.
Experimental studies have shown that Gliatilin stimulates the dose-dependent release of acetylcholine under physiological conditions of neurotransmission.
When ingested, it is broken down by enzymes into choline and glycerophosphate.
Gliatilin, on the one hand, being a choline donor, increases the synthesis of acetylcholine and has a positive effect on neurotransmission, on the other hand, glycerophosphate is involved in the synthesis of phosphatidylcholine (membrane phospholipid), as a result, both have a positive effect on membrane elasticity and receptor function, which improves synaptic transmission.
Thus, pharmacodynamic studies have shown that Gliatilin acts on the synaptic, incl. cholinergic neurotransmission; plasticity of the neuronal membrane; receptor function.
Pharmacokinetics
Absorption when taken orally - 88%; easily penetrates the BBB, accumulates mainly in the brain (concentration in the brain reaches 45% of the level in the blood), lungs and liver; 85% is excreted by the lungs in the form of carbon dioxide, the remaining amount (15%) is excreted by the kidneys and through the intestines.
Does not affect the reproductive cycle, does not have teratogenic or mutagenic effects.
Indications for use Gliatilin 600mg/7ml 7ml 10 pcs. oral solution
- acute period of TBI with a predominantly brain stem level of damage (impaired consciousness, coma, focal hemispheric symptoms, symptoms of brain stem damage);
- ischemic (acute and recovery period) and hemorrhagic stroke (recovery period);
- degenerative and involutional psychoorganic syndromes and consequences of cerebrovascular insufficiency, such as primary and secondary disorders of mnestic functions, characterized by memory impairment, confusion, disorientation, decreased motivation, initiative, and ability to concentrate;
- changes in the emotional and behavioral sphere: emotional lability, increased irritability, decreased interest, senile pseudomelancholy;
- multi-infarct dementia.
Contraindications
Hypersensitivity to the components of the drug.
Application Gliatilin 600mg/7ml 7ml 10 pcs. solution for oral administration during pregnancy and lactation
Contraindicated during pregnancy and lactation.
special instructions
Impact on the ability to drive vehicles and operate machinery
Caution should be exercised when driving vehicles or engaging in other activities that require increased concentration and/or speed of psychomotor reactions.
Overdose
Symptoms: nausea.
If this symptom appears, it is recommended to reduce the dose of the drug.
Side effects Gliatilin 600mg/7ml 7ml 10 pcs. oral solution
Nausea (as a consequence of dopaminergic activation), in this case the dose of the drug should be reduced. Allergic reactions are possible.
As a rule, the drug is well tolerated even with long-term use.
Drug interactions
No drug interactions have been established.
Replacing injections with capsules
When the patient is unable to swallow on his own or his mental state is impaired, due to which he refuses to take pills, intramuscular or drip administration is prescribed. This treatment regimen can be replaced with capsules if the patient is recovering and his swallowing function allows him to take large pieces of food. The same applies to patients in a coma - as soon as the patient regains consciousness and begins to feed on his own, Gliatilin is transferred to capsule form. This allows the patient to go home under the supervision of a local specialist.