Adenoprosin 29 mg 10 pcs. rectal suppositories pharmaprim
pharmachologic effect
A remedy for the treatment of urological diseases.
Composition and release form Adenoprosin 29 mg 10 pcs. rectal suppositories pharmaprim
Suppositories - 1 soup:
- active ingredient: active complex Adenoprosin® 150 mg, calculated as total protein 29 mg;
- excipients: solid fat - Suppotsir AM - sufficient quantity to obtain a suppository weighing 2000.0 mg.
Rectal suppositories, 29 mg.
5 suppositories are placed in a blister pack made of PVC/PE film.
2 blister packs together with instructions for medical use are placed in a cardboard box.
Description of the dosage form
Suppositories are cylindrical-conical, yellow to brown in color. Inhomogeneity of color in the form of inclusions of a darker color is allowed.
Directions for use and doses
Rectally, one suppository 1 time per day (preferably at night at the same time).
The duration of treatment is from 1 to 3 months, depending on the intensity of inflammatory processes in the prostate and the severity of BPH symptoms, as well as their combination.
If necessary, the course of treatment can be repeated.
Pharmacodynamics
The active substance included in the drug ADENOPROSYN® is a biomass obtained from the larvae of insects of the gypsy moth species (Lymantria dispar), which has anti-inflammatory and antioxidant effects.
The biologically active components of the drug reduce the formation of A2-phospholipase and the release of arachidonic acid with a decrease in the synthesis of prostaglandins and leukotrienes (suppress 5-lipoxygenase).
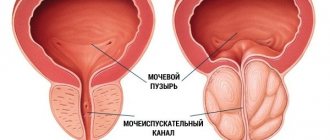
The drug reduces capillary permeability, reduces prostate swelling, and improves microcirculation in the prostate gland.
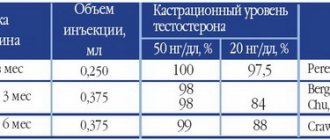
The effect of the drug ADENOPROSIN® is manifested through pathogenetic and nonspecific mechanisms. The drug ADENOPROSIN® already in the first days after the start of use improves urodynamic parameters (increases the value of the maximum volumetric flow rate of urine, reduces urination time, reduces the amount of residual urine) and the general condition of patients with benign prostatic hyperplasia (BPH) and chronic prostatitis (reduces the index of chronic prostatitis, reduces the content of leukocytes in the secretion of the prostate gland, improves the homogeneity of its echostructure).
The drug regulates the tone and peristalsis of the lower segments of the urinary tract, reducing the frequency of urination, including at night, and reducing dysuria, the feeling of incomplete emptying of the bladder and tension during urination.
The antioxidant effect of the drug ADENOPROSYN® is expressed by inhibition of lipid peroxidation due to the antioxidant water-soluble compounds of the drug.
Pharmacokinetics
The effect of the drug ADENOPROSYN® is the combined effect of biologically active components of biomass obtained from the larvae of insects of the species Lymantria dispar, so pharmacokinetic studies are not currently possible.
Indications for use Adenoprosin 29 mg 10 pcs. rectal suppositories pharmaprim
Benign prostatic hyperplasia.
Chronic prostatitis (in combination therapy).
Contraindications
Hypersensitivity to the components of the drug; acute urinary retention; children under 18 years of age; malignant neoplasms of the prostate gland.
Application Adenoprosin 29 mg 10 pcs. rectal suppositories pharmaprim during pregnancy and breastfeeding
The drug is not used in women and children under 18 years of age.
special instructions
The drug should be used after bowel movements or an enema.
It is recommended that after administration of the drug the patient remains in a lying position for 30-40 minutes.
Impact on the ability to drive vehicles and operate machinery
The drug does not affect the ability to drive vehicles or operate machinery.
Overdose
To date, no cases of overdose have been registered.
Side effects Adenoprosin 29 mg 10 pcs. rectal suppositories pharmaprim
From the gastrointestinal tract: rarely - diarrhea or frequent stools; itching in the anal area.
Allergic reactions and general weakness are possible.
Drug interactions
To date, no cases of clinically significant drug interactions between ADENOPROSYN® and other drugs have been reported.
Adenoprosin 250 mg No. 10 sup. rect.
Instructions for the use of the drug for specialists ADENOPROSYN Trade name Adenoprosin International nonproprietary name No Dosage form Rectal suppositories Composition One suppository contains the active substance - adenoprosin 250 mg, excipients: p-hydroxybenzoic acid methyl ester (nipagin), p-hydroxybenzoic acid propyl ester (nipazole ), semi-synthetic glycerides. Description Suppositories are yellow to brown in color, cylindrical-conical in shape with a glossy and/or matte surface. Inhomogeneity of color in the form of inclusions of a darker color is allowed. The cut is allowed to have an airy and porous rod and a funnel-shaped depression. Pharmacotherapeutic group Other drugs for the treatment of benign prostatic hyperplasia. ATC code G04CX Pharmacological properties Pharmacokinetics The drug is of entomological origin and its action is the combination of all active components, a lipoprotein extract obtained from the larvae of insects of the Lepidoptere species, therefore pharmacokinetic studies are not possible. Pharmacodynamics Lipoprotein extracted from the larvae of insects of the Lepidoptere species has an antioxidant, vasoprotective, anti-inflammatory and pronounced immunomodulatory effect. Biologically active components reduce the formation of A2 phospholipase and the release of arachidonic acid with a decrease in the synthesis of prostaglandins and leukotrienes (suppresses 5-lipoxygenase). The drug reduces vascular permeability and reduces swelling. Thanks to pathogenetic and nonspecific mechanisms, Adenoprosin improves urodynamic parameters and the condition of patients with benign prostatic hyperplasia (BPH) and chronic prostatitis. The antioxidant effect of Adenoprosin is due to inhibition of lipid peroxidation due to antioxidant water-soluble compounds. Indications for use - benign prostatic hyperplasia (grades I-II) - chronic prostatitis Method of administration and dosage Rectally, one suppository once a day (preferably at night at the same time). The drug should be used after bowel movements or an enema. After administering the suppository, it is recommended that the patient remain in a lying position for 30-40 minutes. The duration of treatment is from 1 to 3 months, depending on the intensity of inflammatory processes at the prostate level and the severity of symptoms of prostate adenoma, as well as their combination. If necessary, the course of treatment can be repeated. Side effects and reactions - general weakness Rarely - diarrhea Contraindications - prostate cancer - acute urinary retention - hypersensitivity to the components of the drug Drug interactions No clinically significant interactions were observed. Special instructions Features of the drug's influence on the ability to drive a vehicle or potentially dangerous mechanisms The drug does not affect the ability to drive a vehicle or potentially dangerous mechanisms. Overdose No cases of drug overdose have been observed. Release form Rectal suppositories 250 mg. 5 suppositories each in a polyvinyl chloride blister pack. 2 blister packs along with instructions for use are placed in a cardboard box. Storage conditions Store in a dry place, protected from light, at a temperature from +2˚С to +8˚С. Keep out of the reach of children! Shelf life: 1.5 years Do not use after the expiration date indicated on the package! Conditions for dispensing from pharmacies By prescription Manufacturer Farmaprim SRL for ICS Insect-Farm SRL Republic of Moldova, Chisinau, st. G. Tudor, 3.