Pharmacological properties of the drug Primolut-nor
Complete endometrial transformation can be achieved with a total dose of 30–60 mg of norethisterone acetate for 10 days in post-castration women with adequate estrogen supplementation. This amount is sufficient to achieve the endometrial state corresponding to the completion of the luteal phase of the cycle. Menstrual-like withdrawal bleeding usually begins 2–4 days after stopping the drug. Thus, like all sex hormones, norethisterone acetate inhibits the secretion of gonadotropins in the anterior pituitary gland: taking 5 mg of norethisterone acetate daily from the 5th day of the cycle can lead to suppression of ovulation. Like progesterone, norethisterone acetate increases basal body temperature: taking 5 mg of norethisterone acetate daily increases basal body temperature by approximately 0.5 °C. In addition to its transformative effect, norethisterone acetate has a hemostatic effect. Local effect on the endometrium leads to the cessation of dysfunctional bleeding. Following oral administration of a range of doses (0.3–25 mg) of norethisterone acetate is rapidly and completely absorbed. After absorption and the first passage through the liver, norethisterone acetate undergoes hydrolysis to form norethisterone, the active substance, and acetic acid. The maximum concentration of norethisterone in blood plasma is achieved approximately 2 hours after administration. Concentrations decrease biphasically with half-lives of 1–3 hours and approximately 10 hours. These values remain stable after repeated dosing over several months. Plasma concentrations vary between individuals due to individual differences in hepatic clearance and sex hormone binding globulin (SHBG) concentrations; about 35% of norethisterone binds to SHBG, 61% to albumin. Accordingly, 3–4% of norethisterone in blood plasma is detected in unbound form. After taking one tablet of Primolut-Nor, the maximum plasma concentration of norethisterone reaches approximately 18 ng/ml (25 ng/ml). Research results have shown partial conversion of norethisterone acetate to ethinyl estradiol during metabolism. Oral administration of 1 mg of norethisterone acetate produces an amount of ethinyl estradiol equivalent to a human oral dose of approximately 6 mcg. Due to metabolic processes during the first passage through the liver, the absolute bioavailability of norethisterone is about 60%. However, significant changes are possible. Drugs that induce liver enzymes may reduce bioavailability. Norethisterone penetrates the BBB and the placental barrier. Norethisterone is not excreted unchanged. After biotransformation, metabolites are formed due to reduction of the aromatic ring and hydroxylation, as well as their conjugates (glucuronides and sulfates). A minority of metabolites with very good solubility in water are slowly eliminated from the blood plasma (half-life 42–67 hours). Excretion of metabolites in urine and feces occurs in a ratio of 6:4. The largest portion is excreted with a half-life of 1 day.
Use of the drug Primolut-nor
Before using the drug Primolut-Nor, it is necessary to conduct a thorough general medical and gynecological examination (including mammary glands and cytological examination of a smear from the cervix and cervical canal) and exclude the presence of pregnancy. In case of prolonged use of the drug Primolut-Nor, a control examination should be carried out every 6 months for the purpose of prevention. The tablets are taken without chewing, washed down with liquid. It is recommended to adhere to the following rules of use: Dysfunctional bleeding For 10 days, 2 times a day, take 1 tablet of the drug Primolut-Nor, which will lead in 1-4 days to the cessation of uterine bleeding not caused by an organic disease. Sometimes in the first days after starting to use the drug, bleeding decreases and only after 5-7 days it stops completely. Successful treatment allows continued regular use of the drug Primolut-Nor even after the bleeding has stopped (a total of 20 tablets of the drug Primolut-Nor, 5 mg each). 2-4 days after stopping the use of the drug, due to the lack of entry into the body of the drug, withdrawal bleeding occurs, the intensity and duration of which is similar to normal menstruation. Light bleeding while taking the tablets Sometimes minor spotting is noted after the initial cessation of bleeding. In such cases, you should not stop taking the pills. Lack of hemostasis, intense uterine bleeding In the case of uterine bleeding, the use of the drug Primolut-Nor allows one to draw important differential diagnostic conclusions. If bleeding does not stop with regular use of the tablets, this indicates an organic or extragenital nature of the origin of the bleeding (for example, polyps, cervical or endometrial cancer, fibroids, incomplete abortion, ectopic pregnancy, thrombcytopenia, thrombasthenia). The same applies to those cases when, even during the period of taking the drug after the initial cessation of bleeding, new intense bleeding is noted. Prevention of relapses To avoid relapses of dysfunctional bleeding, it is recommended to take the drug Primolut-Nor over the next three cycles. This applies only to those cases where the basal body temperature curve, which must be measured regularly, indicates the presence of a monophasic cycle and, taking this into account, the threat of follicle persistence and its consequences. From the 19th to the 26th day of each cycle, 1 tablet of Primolut-Nor 2 times a day (1st day of the cycle = 1st day of the last bleeding). Withdrawal bleeding associated with discontinuation of the drug begins a few days after taking the last tablet. Primary and secondary amenorrhea In the case of secondary amenorrhea, hormonal therapy is carried out no earlier than 8 weeks after the last menstruation. In order to achieve menstrual-like bleeding in combination with the use of the drug Primolut-Nor, it is necessary to prescribe estrogen. Before starting therapy, it is necessary to exclude the presence of a pituitary tumor that produces prolactin, since the possibility of growth of macroadenomas cannot be excluded under the condition of prolonged exposure to increased doses of estrogens. Exception: this therapy is not prescribed to patients for whom it has been clearly established that there is no sufficient formation of their own estrogens (primary amenorrhea caused by gonadal dysgenesis). Attention . In order to prevent pregnancy, it is necessary to use non-hormonal methods of contraception (with the exception of calendar and temperature methods). If, while following the above-mentioned regimen of continuing therapy approximately every 28 days, there is no withdrawal bleeding associated with a break in the use of the drug, the possibility of pregnancy should be assumed, despite the use of preventive measures. In this case, you should take a break from treatment until the results of the differential diagnosis are obtained. Premenstrual syndrome, mastopathy Taking 1 tablet of Primolut-Nor 1-2 times a day from the 19th to the 26th day of the cycle eliminates or minimizes the phenomena that occur during the premenstrual period: headache, depressed mood, fluid retention, mastodynia. Shifting the time of the onset of menstruation Due to special reasons, it may be necessary to shift the time of the onset of monthly bleeding to an earlier or later date. If bleeding shifts to an earlier date, use drugs that are a combination of gestagens and estrogens. Due to their ability to delay ovulation, they virtually eliminate the possibility of pregnancy. If it is necessary to shift the time of menstruation to a later date, the use of the drug Primolut-Nor is possible only if pregnancy is excluded, which can be problematic, since this drug must be taken at a time when, based on existing examination methods, it is impossible to clearly exclude pregnancy. Therefore, the use of Primolut-Nor is limited to cases where pregnancy in the first trimester is excluded in a given cycle. Dosage: for no more than 10–14 days, 2 times a day, 1 tablet of Primolut-Nor, starting approximately 3 days before the start of the expected menstruation. Bleeding occurs 2-3 days after stopping taking the pills. Endometriosis The course of treatment begins on the 5th day of the cycle with 1 tablet of Primolut-Nor taken 2 times a day. If bleeding occurs, it is necessary to increase the dose and take 2 tablets of the drug twice a day. After bleeding stops, you can reduce the dose to the initial dose. The duration of treatment is at least 4–6 months. During treatment, ovulation and menstruation are absent. After completion of hormonal therapy, withdrawal bleeding occurs due to discontinuation of the drug.
Hormonal preparations Bayer Primolut-Nor tablets 5 mg No. 60
Good afternoon everyone.
And finally I got to the review of this drug.
My whole saga with hormone therapy is over.
And now I have the time and energy to write reviews about the drugs that were used in my treatment.
I'll start with the first of them, BUT
I’ll write right away that this review will be completely honest...
This review is based ONLY on my experience with use and in no way means that this drug will affect you in the same way!
If your doctor prescribes it for you, do not be afraid, but drink... In cases of side effects like mine, you can always call your doctor and change or stop the drug!
Primolut-Nor.
Or Primolut-N (in Europe).
Let me start with the fact that my husband and I have been wanting a baby for a long time... but, alas, it hasn’t worked out yet.
And so we turned to a private doctor, the best and most expensive one in our city...
Everyone almost calls him “a doctor from God.” But, alas, not in our case yet...
So I’ll go back to how I came to treatment with this drug...
My story began in the fall of 2014, with this particular doctor (before that there were other doctors).
The doctor talked with us, carried out all the examinations and looked at all the tests prescribed for me, carried out certain hormonal therapy, after which there was supposed to be a procedure with the help of which I was supposed to have a long-awaited pregnancy... but, alas, this did not work out, and besides, I got I have a bad hormonal imbalance, as a result of which I got something like amenorrhea.
At first I lost my period for 2 months, but after drinking vitamin E my cycle was restored. But he recovered only for 2 months. After two normal cycles, in the third month my periods disappeared again.
Moreover, this time for exactly six months... At the same time, I had no discharge at all. There was only PMS cider. After the New Year, as scheduled, PMS and slight spotting began again, after which I waited for my period, but it never came (I’ll say right away that my cycle was always unstable and always came with delays). And besides, just a couple of weeks after the discharge ended, it started again. Then I decided that I needed to go to the doctor.
At that time, I trusted this doctor, because during the previous hormone therapy I did not have any side effects or complaints about the treatment or my health. Everything began to manifest itself after hormone therapy.
After an examination, the doctor diagnoses me with amenorrhea due to hormonal therapy (hormonal imbalance), followed by dysfunctional uterine bleeding in the form of small spotting.
And in order to try again to get pregnant and to start new hormone therapy, he prescribes me the drug Primolut-Nor.
Primolut was supposed to stop the discharge, and then when I stopped the drug, I was supposed to have my period.
After which, to restore the cycle, I was prescribed contraceptives. And then tests and hormone therapy.
And so, having bought the pills and coming home, I began to study information about the drug.
I will say right away that the reviews for this drug are basically positive.
And I was not prepared for what I encountered.
I trusted the doctor and, of course, people’s reviews of this drug.
And now about the tablets themselves:
Primolut-Nor:
Some information and instructions:
pharmachologic effect
A gestagenic agent causes the transformation of the uterine mucosa from the proliferation phase to the secretory phase, and after fertilization creates the necessary conditions for the implantation of a fertilized egg. Reduces the excitability and contractility of the uterine muscles, stimulates the development of the end elements of the mammary gland. Suppresses the secretion of gonadotropins by the pituitary gland, preventing follicle maturation and ovulation.
Indications
Premenstrual syndrome, anovulatory metrorrhagia, infertility, miscarriage, dysmenorrhea (accompanied by shortening of the secretory phase), algomenorrhea, amenorrhea, endometriosis, adenomyosis, fibroids, mastodynia, mastopathy, glandular cystic endometrial hyperplasia, endometrial cancer, menopausal syndrome, diagnostic progesterone test; contraception, cessation and prevention of lactation, postponing the next menstruation.
Contraindications
Hypersensitivity, breast cancer (including suspected), pregnancy (including suspected, except for cases of use for miscarriage), cholestatic jaundice of pregnant women in history, jaundice (including in history), acute liver diseases (including benign and malignant neoplasms), acute thrombophlephitis or thromboembolism, bleeding from the urinary tract of unknown origin, metrorrhagia of unknown origin, bleeding from the genitals of unknown origin, puberty. With caution. Bronchial asthma, CHF, epilepsy, arterial hypertension, migraine, renal failure, convulsions or other dysfunctions of the central nervous system (including a history), diabetes mellitus, liver disease (history), hyperlipidemia, history of thrombophlebitis, thromboembolism in medical history.
Side effects
Nausea, vomiting, metrorrhagia, peripheral edema, allergic reactions (skin rash, itching), paresthesia, weight gain, increased fatigue, engorgement of the mammary glands. With long-term use - thrombosis, thromboembolism.
My package of tablets consisted of 30 pieces.
I should have taken them for 6 days, 3 pieces per day, to completely stop the discharge.
If the discharge does not stop, then call the doctor and then the doctor will prescribe further use of the drug or its replacement.
The tablets themselves are small in size. No smell or taste.
And now the most interesting thing is how I felt all the side effects of these pills:
The first day, I didn’t have any side effects.
On the contrary, I became more active, which surprised me.
But then, I felt all the charm of the pills...
Weight gain
Due to fluid retention in the body. I swelled like I had never swelled before. Swelling is my eternal problem during PMS, I always become bloated... But here it was something with something.... The face puffed up terribly... 2 cm plus in the waist, stomach and hips....
Fatigue
I couldn’t help myself, I lay around all day….I tried really hard to do at least something, plus at least do a little sports, at least do exercises….
Breast enlargement
My breasts have gotten bigger, they always get bigger during PMS, but then they started to hurt terribly. It was impossible to touch.
I have already started counting the days until the end of taking this drug and began to pray that my critical days would come and all this would pass!!!
The veins in my arms began to swell, and it looked very terrible... Increased appetite
This appeared on the 3rd day of taking the drug, and my appetite was such that anyone would envy.
Not an appetite, but a glutton and a wild one!!!
But thanks to proper nutrition and a certain rhythm of life, I did not gain weight, almost, but only swelled, and still the pills did the job +1 kg.
I restrained myself as best I could, but I just wanted so much sweets. I ate a little in the morning and endured, endured, endured...
Depression
This is perhaps the most scary point for me. However, the instructions say nothing about it!
Because when taking these pills, I began to experience depression, which was aggravated by contraceptives in the subsequent use after this drug...
I didn’t want anything but to go somewhere. It seemed to me. that no one loves me and that I’m unhappy... But it turns out they were flowers... berries were waiting for me ahead...
But no matter what, I took this drug for all 6 days.
My bleeding stopped, by the way, on the third day of taking it. But I took the entire course prescribed by the doctor. After which the doctor said that he would not extend the course and we would not switch to another drug... now withdrawal bleeding or critical days should occur in 2-4 days.
BUT, they did NOT come!!!
The drug did not help me!!!
It was necessary to call for critical days to be canceled in a different way.
After which I started a course of taking contraception, which worsened my condition and depression.
Result:
Take this drug only as directed and under the supervision of your doctor.
Based on my condition when taking it, I do not want and will not take this drug again.
If there is an opportunity to replace it, I always use it. But again, with the doctor’s permission, which is very important!
I may have added a fly in the ointment with this review of this drug, but I think it’s important.
One drug does not always affect everyone the same. And this is probably the first or second negative review about this drug.
I cannot advise him or not.
ONLY your attending physician can do this!
Thank you for your attention.
If you are interested, you can read my reviews about other drugs:
Contraceptives Yarina is a contraceptive. Prescribed for me to restore my cycle after Primolut or the story of how my husband and I almost divorced….
Vitamin E - restores and normalizes the cycle, as well as for nails and hair....
Vitamins B-Complex “50” - for stress, nervous disorders and dietary nutrition.
Homeopathy Edas 306 - against aggression and nervous breakdowns
Blefarogel 1
Phosphalugel - helped me heal my stomach. The best assistant to this day.
Glycine Bio - helped 100% with my insomnia.
Enterosorbent Polyphepan - for problem skin, poisoning and hangover.
Special instructions for the use of the drug Primolut-nor
If the patient has diabetes mellitus, the doctor must be informed about this, since in this case the patient needs constant monitoring during therapy. Research results have shown that the administration of oral medications containing estrogens and gestagens that delay ovulation is accompanied by a more frequent occurrence of thromboembolic complications. Therefore, the possibility of an increased risk of thromboembolism should be taken into account in the presence of a history of thromboembolic diseases, severe diabetes with vascular damage, or sickle cell anemia. In isolated cases, after the use of hormones included in Primolut-Nor, the formation of benign liver tumors was observed, and in exceptional cases - malignant. In isolated cases, these tumors have led to life-threatening intra-abdominal bleeding. If there is pain in the upper abdomen, liver enlargement, or signs of intra-abdominal bleeding, the differential diagnosis should take into account the possibility of a liver tumor. Reasons for immediate discontinuation of the drug Migraine-like headache that has not been noted before or an increase in the frequency of unusually severe headaches; sudden changes in vision or hearing; the first signs of thrombophlebitis or symptoms of thromboembolism (for example, unusual pain in the legs or swelling of the legs, stabbing pain in the chest for no apparent reason when breathing or coughing); feeling of pain and tightness in the chest; planned operations (6 weeks before their implementation); prolonged immobilization (for example, as a result of injury); manifestations of jaundice; hepatitis A; skin itching; a sharp increase in blood pressure; pregnancy. During pregnancy and breastfeeding. The use of Primolut Nor is contraindicated during pregnancy. Up to 0.1% of the daily dose of norethisterone is excreted in breast milk. impact on the ability to drive vehicles and operate machinery .
Primolut-Nor tablets 5 mg, 30 pcs.
To prevent pregnancy, it is necessary to use non-hormonal methods of contraception (barrier).
Before starting or continuing treatment with the drug Primolut-Nor, an individual assessment of the benefit/risk ratio should be carried out if any of the disorders/risk factors listed below exist.
Vascular disorders
Based on epidemiological studies, it has been established that oral administration of ovulation inhibitors containing estrogens/progestogens leads to an increase in the incidence of thromboembolic disorders. Therefore, it is necessary to take into account the possibility of an increased risk of thromboembolism, especially if there is a history of such diseases.
Generally accepted risk factors for venous thromboembolism (VTE) are: personal or family history of the disease (VTE in a sibling or parent at a relatively early age), age, obesity, prolonged immobilization, major surgery, severe trauma.
The increased risk of thromboembolism during the postpartum period should be taken into account.
Treatment should be stopped immediately if symptoms of arterial or venous thrombosis appear or if it is suspected.
Patients with a history of VTE or a known thrombotic condition are at increased risk of developing VTE. Treatment with steroid hormones may increase this risk. Patients with a personal or family history of thromboembolism or recurrent spontaneous abortions should be evaluated to rule out thromboembolism. Patients receiving anticoagulant therapy should be carefully assessed regarding thromboembolic risks before initiating treatment with progestogens. In cases of prolonged immobilization, elective surgery, especially in the abdominal region, or orthopedic surgery on the lower extremities, it is necessary to discontinue progestin therapy 4-6 weeks before surgery. Continuation of treatment with progestogens is possible only after complete restoration of motor activity.
Tumors
Isolated cases of benign liver tumors have been described, and even more rarely, cases of malignant tumors in patients taking hormonal substances included in the drug Primolut-Nor. In some cases, these tumors have resulted in life-threatening intra-abdominal bleeding.
In women taking COCs, benign liver tumors have been reported in rare cases and malignant liver tumors have been very rarely reported. In rare cases, these tumors have led to intra-abdominal bleeding that is life-threatening. If women receiving COCs experience severe pain in the upper abdomen, signs of liver enlargement, or signs of intra-abdominal bleeding, it is necessary to differentiate a liver tumor.
Other conditions
Patients with diabetes should be under medical supervision.
In rare cases, chloasma may occur, primarily in women with a history of chloasma during pregnancy. Women prone to chloasma should avoid exposure to the sun or ultraviolet rays while taking Primolut-Nor.
In acute visual impairment, exophthalmos, diplopia or migraine, papilledema or retinal lesions should be excluded.
Progestogens can cause fluid retention. Prescribe with caution to patients with epilepsy, migraine, asthma, cardiac dysfunction.
When using COCs in women with hypertriglyceridemia or a family history of it, there may be an increased risk of developing pancreatitis.
Patients with a history of depression should be closely monitored by doctors. It is necessary to stop taking the drug if depression progresses.
Medical examination, examination and consultation with a doctor
Before starting or resuming treatment with Primolut-Nor, it is necessary to collect a complete medical history (including family history), the woman must undergo a full medical examination, including a gynecological examination: contraindications should be taken into account (see Section “Contraindications”) and application features (see section "Peculiarities of use") of the drug. The study must be repeated periodically during treatment with Primolut-Nor. The frequency and type of these studies depend on the individual characteristics of each woman, but they must necessarily include blood pressure measurement, examination of the mammary glands, abdominal and pelvic organs, as well as cytological examination of the cervix.
Reasons for immediate cessation of treatment
Initial onset of severe headaches and migraines or an increase in the frequency of unusually severe migraines, sudden disturbances in perception (for example, blurred vision or hearing), first signs of thrombophlebitis or symptoms of thromboembolism, sensation of pain and tightness in the chest, planned surgical procedures (6 weeks before surgery ), immobilization, the appearance of jaundice, the development of hepatitis (non-icteric), generalized itching, a significant increase in blood pressure, pregnancy.
Additional caveats based on partial conversion of norethisterone to ethinyl estradiol
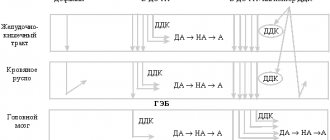
Norethisterone is metabolized partially to ethinyl estradiol, that is, for each mg of norethisterone/norethisterone acetate taken orally, an amount of ethinyl estradiol is formed equal to the oral dose for humans of approximately 4-6 mcg.
Due to the partial conversion of norethisterone to ethinyl estradiol, it is believed that the use of Primolut-Nor may cause pharmacological effects similar to those of combined oral contraceptives (COCs). Therefore, the following general precautions should be taken into account when using COCs.
Vascular disorders
The risk of VTE is highest during the 1st year of use. This increased risk occurs after COCs are started for the first time or again (after at least 1 month or more of non-use).
Epidemiological studies have shown that the incidence of VTE in patients taking low estrogen COCs (<50 mcg ethinyl estradiol) is approximately 20 to 40 cases per 100,000 woman-years, but the estimated risk varies depending on the progestin. These data can be compared with 5-10 cases per 100,000 woman-years in people not taking oral contraceptives. The use of any COC is associated with an increased risk of VTE compared with that in the case when they are not used. This increased risk is less than the risk of pregnancy-associated VTE, which is estimated to be 60 per 100,000 pregnancies.
VTE can be life-threatening and may be fatal (in 1-2% of cases).
VTE, which is manifested by deep vein thrombosis and/or pulmonary embolism, can occur when taking any COC.
Very rarely, thrombosis of other blood vessels, such as the arteries and veins of the liver, kidneys, mesenteric vessels, veins and arteries of the brain or retina, has been reported in women using COCs.
COC use is associated with an increased risk of acute myocardial infarction (AMI) or stroke, which risk is largely dependent on the presence of other risk factors.
Common signs/symptoms of arterial or venous thrombotic/thromboembolic events or stroke may include:
- Severe pain in the tibia of one leg; swelling of the lower leg;
- Sudden severe pain in the chest with possible radiation to the left arm;
- Sudden shortness of breath;
- Sudden attack of coughing;
- Any unusual, severe or prolonged headache;
- Sudden partial or complete loss of vision;
- Double vision;
- Vague broadcasting or aphasia
- Dizziness;
- Collapse with or without focal epilepsy;
- Weakness or sudden numbness of one side or part of the body;
- Movement disorders;
- Acute stomach.
A possible increased synergistic risk of thrombosis should be taken into account in women with a combination/combination of multiple risk factors or a single major risk factor. The increase in risk may be greater than the sum of the risks associated with each individual factor. If the benefit/risk ratio is unfavorable, COCs should not be prescribed (see section "Contraindications").
The risk of developing venous or arterial thrombotic/thromboembolic events or stroke increases with the presence of the following factors:
- Age;
- Obesity (body mass index more than 30 kg/m2);
- Relevant family history (i.e., siblings or parents having venous or arterial thromboembolism at a relatively early age). If a hereditary predisposition is suspected, the woman should be referred to an appropriate specialist for consultation before deciding to use COCs;
- Prolonged immobilization, major surgery, any surgery on the lower extremities or major trauma. In these situations, it is advisable to stop using COCs (in the case of elective surgery at least 4 weeks before the intervention) and not restart the drug until remobilization;
- Smoking (with heavy smoking and with age, the risk increases, especially in women over 35 years of age);
- Dyslipoproteinemia;
- Arterial hypertension;
- Migraine (an increase in the frequency and severity of migraine during the use of COCs may be a prodrome of cerebrovascular accidents and, therefore, is grounds for immediate cessation of COC use);
- Heart valve pathology
- Atrial fibrillation.
There is no consensus regarding the possible influence of varicose veins and superficial thrombophlebitis on the development of VTE.
Other conditions associated with adverse circulatory effects include diabetes mellitus, systemic lupus erythematosus, hemolytic uremic syndrome, chronic inflammatory bowel disease (Crohn's disease and ulcerative colitis), and sickle cell anemia.
An increase in the frequency or severity of migraine during the use of COCs may be a prodrome of cerebrovascular accidents and, therefore, is grounds for immediate discontinuation of COC use.
Biochemical factors that may indicate hereditary or acquired predisposition to venous or arterial thrombosis include activated protein C (APC) resistance, hyperhomocysteinemia, antithrombin III deficiency, protein C deficiency, protein S deficiency, antiphospholipid antibodies (anticardiolipin antibodies, lupus anti- coagulant).
When assessing the benefit/risk ratio of a drug, the physician should remember that proper management of the patient can reduce the relative risk of thrombosis and the risk of VTE associated with pregnancy is higher than the risk associated with the use of low-dose COCs (˂0.05 mg ethinyl estradiol).
Oncological diseases
The most important risk factor for the development of cervical cancer is the persistence of human papillomavirus (HPV) infection. The results of some epidemiological studies indicate an increased risk of cervical cancer with long-term use of COCs. However, this statement remains controversial, since it is not clear to what extent the study results take into account associated risk factors, such as high frequency of cervical screening, sexual behavior, including the use of barrier contraceptive methods.
A meta-analysis of 54 epidemiological studies suggests a slight increase in the relative risk (RR = 1.24) of developing breast cancer in women using COCs. This increased risk gradually disappears over 10 years after stopping COC use. Because breast cancer is rare in women under 40 years of age, the increase in breast cancer incidence in women who are current or recent users of COCs is small compared with the overall risk of breast cancer. The results of these studies do not provide evidence for a causal relationship. The identified increased risk may be due to both earlier diagnosis of breast cancer in women using COCs, the biological effects of COCs, or a combination of both factors. It has been noted that breast cancer detected in women who have ever taken COCs is, as a rule, clinically less severe than in those who have never used COCs.
Neoplasms can be life-threatening or fatal.
Other conditions
Although slight increases in blood pressure have been reported in many women taking COCs, clinically significant increases are rare. However, if persistent clinically significant hypertension develops while taking a COC, the physician should discontinue the COC and begin treatment for hypertension. If normal blood pressure levels are achieved after antihypertensive therapy, COC use can be reinstated if deemed appropriate.
The occurrence or exacerbation of the following diseases has been reported during pregnancy and with the use of COCs, but their connection with the use of COCs has not been conclusively proven: jaundice and / or itching associated with cholestasis; formation of gallstones; porphyria; systemic lupus erythematosus, hemolytic-uremic syndrome, Sydenham's chorea; herpes of pregnancy hearing loss associated with otosclerosis.
In women with hereditary angioedema, exogenous estrogens may cause or exacerbate symptoms of the disease.
Acute or chronic liver dysfunction may require discontinuation of COCs until liver function tests normalize. Recurrence of cholestatic jaundice, first manifested during pregnancy or previous use of sex steroids, requires discontinuation of COC use. Crohn's disease and ulcerative colitis have been associated with COC use.
Impact on laboratory results
Taking progestogens may affect the results of some laboratory tests.
Increased ALT levels
In clinical trials in patients receiving therapy for the treatment of hepatitis C virus with drugs containing ombitasvir/paritaprevir/ritonavir and dasabuvir with or without the addition of ribavirin, transaminase (ALT) levels increased more than 5 times the upper limit of normal (ULN) observed significantly more frequently in women who used medicinal products containing ethinyl estradiol, such as combined hormonal contraceptives (CHCs). Since norethisterone is partially metabolized to ethinyl estradiol, this caution applies to women taking norethisterone (see Sections “Contraindications” and “Interaction with other drugs and other types of interactions”).
Cautions regarding excipients
This medicine contains lactose. Patients with hereditary forms of galactose intolerance, Lapp lactase deficiency or poor absorption of glucose or galactose should not use this drug.